 |
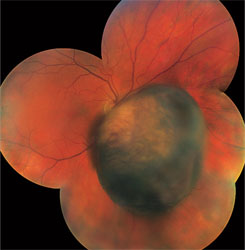
| Figure 1. Typical appearance of a medium-sized choroidal melanoma. While most are dome-shaped, this one has developed a collar-button configuration due to a rupture in Bruch's membrane. This appearance is virtually pathognomonic for choroidal melanoma. |
Choroidal and ciliary body melanoma is the most common primary ocular malignancy and therefore is an important problem for ophthalmologists (See Figure 1). The extensive body of literature in part reflects this. A Pubmed search of uveal melanoma yields more than 3,500 citations during the last 50 years. This body of work documents significant advances. In this article, I will provide an overview of many of these advances and provide insight into what we may expect in the coming years.
Background
Histopathologic studies in the 1960s and 1970s from the Armed Forces Institute of Pathology demonstrated that approximately 20 percent of enucleated eyes submitted with a presumed diagnosis of melanoma were incorrect.1,2 These studies advanced our knowledge of common mimicking lesions. Concurrently, advances in ultrasonography enhanced our ability to differentiate many of these lesions (See Figure 2). Moreover, a shift in philosophy occurred such that eyes with lesions in which the diagnosis was not completely certain were watched (a move away from, “when in doubt, take it out”). Today, experienced examination with indirect ophthalmoscopy and skilled ultrasonography yields an accurate diagnosis, in the absence of biopsy for tissue, in more than 99 percent of cases.3
In the 1980s, the Collaborative Ocular Melanoma Study was undertaken to evaluate two questions: 1) For medium-sized melanoma, is survival better with iodine-125 plaque irradiation or enucleation?2) For a large melanoma, does pre-enucleation low-dose irradiation (20 Gy) reduce the risk of micrometastatic spread induced by the manipulation of the eye during enucleation? These large, prospective, randomized clinical trials provided a wealth of important data.
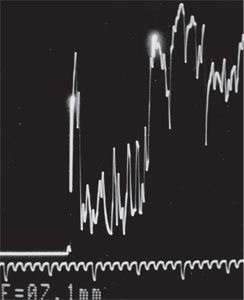 |
| Figure 2. An A-scan ultrasound showing the typical low, homogenous reflectivity pattern consistent with choroidal melanoma. While other lesions can have this reflectivity pattern (for instance, recent choroidal hemorrhage), this pattern is most typical of melanoma and helps to differentiate a choroidal lesion from other tumors such as a choroidal hemangioma or a choroidal metastasis. |
However, despite significant advances in our understanding of the diagnosis and treatment of choroidal melanoma, survival has not improved. This is in stark contrast to the management of many other cancers. Numerous clinical and histophathologic studies have shown that the largest tumor dimension and location are key clinical determinants of survival. Therefore, it seems logical to conclude that earlier intervention and treatment may reduce metastasis of melanoma. Efforts to improve survival have mostly focused on earlier diagnosis. But the question remains as to when we should intervene. And should we intervene in all cases?
Intervention Questions
The diagnosis of a suspicious nevus raises the possibility that the lesion is not a nevus but rather a small growing melanoma. Since choroidal nevus is relatively common (about 4 to 8 percent of eyes), whereas melanoma much more rare, clearly the answer is not to treat all nevi preemptively. On the other hand, perhaps there are some that should be treated (See Figures 3a and b).
Studies evaluating small “suspicious” nevi have attempted to elucidate features that suggest a higher likelihood of continued growth. Small melanocytic lesions are typically less than 10 mm in diameter and 2.5 to 3 mm in thickness. While growth is taken to be a biologic indicator of malignant potential, growth alone is not predictive in all cases. Histopathologically proven choroidal nevi can show some growth. Typically, this is a small amount of growth over many years. In other cases, sufficient growth over months is concerning and typically taken to indicate a need for treatment. As I will review below, new genetic testing suggests that many histopathologically proven melanomas may be at extremely low risk of spreading, confounding this conclusion.
Features of small melanocytic lesions that have been shown to be helpful in evaluating risk of growth include: presence of subretinal fluid; orange lipofuscin pigment; symptoms; peripapillary location and thickness. Other features such as the presence of drusen or significant retinal pigment epithelial clumping are suggestive of a lower risk of growth. Evaluation of a lesion requires careful documentation of the size with color photography and a fundus drawing, and ultrasonography. Subsequent periodic evaluations are essential to detect growth of the lesion that would require treatment. While these higher-risk features are helpful in counseling the individual and determining subsequent follow-up intervals, they are imperfect and not absolutely predictive.
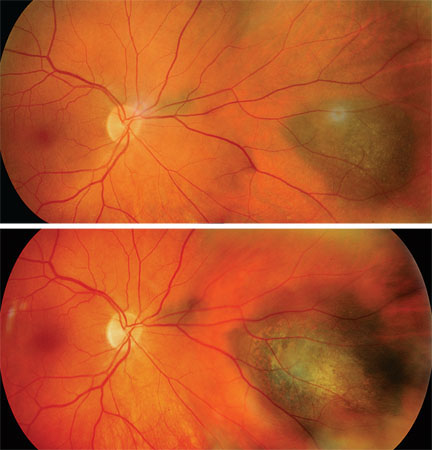 |
| Figure 3a (top). Fundus photography showing a small, suspicious choroidal nevus in the nasal mid-periphery. There is an incidental smaller nevus immediately adjacent to the nerve. 3b. Four years later, note the enlargement of the lesion. It is thicker and has developed a significant orange lipofuscin pigment. At this point, this lesion was treated with transpupillary thermotherapy, and the patient is alive and well with more than 10 years of follow-up and 20/20 vision. |
Abnormalities on chromosomes 1, 3, 6, 8, 11 and 13 have been documented. Tissue can be obtained from enucleated or resected tumors, or alternatively, fine-needle aspiration biopsy techniques at the time of globe-preserving radiation treatment. Of these chromosomal abnormalities, monosomy of chromosome 3 has been shown to be very predictive of a greater risk of metastasis. Another avenue of development has been gene expression profiling of RNA from the primary tumor. This work has been able to classify risk into two classes, one with a very low risk (Class 1), and one with a very high risk of metastasis (Class 2). Availability of this highly predictive test enables us to better treat, manage and monitor our patients with ocular melanoma. In those cases when a patient is found to have a high risk of metastasis, more frequent and extensive monitoring can be undertaken with the goal of earlier detection of metastasis. Furthermore, with the advent of newer drugs for treatment of metastasis, these high-risk patients can be enrolled in treatment trials for prophylaxis against metastasis.
Prophylactic treatment of high-risk patients requires proven and effective treatment strategies. Interferon treatment is an accepted approach but, unfortunately, has demonstrated only marginal benefits. Large prospective trials of patients with cutaneous melanoma have, in general, shown some improvement in disease-free survival, but very modest benefit in overall survival. These results appear to show more favorable outcomes in patients with lower tumor burdens. Therefore, it is logical to consider prophylactic interferon in patients with high-risk uveal melanoma (Study NCI-2010-00640). Investigation of melanoma tumor cell biology guided by insights from gene studies offers hope of development of innovative and more targeted treatments.
New Directions
Ophthalmologists are very familiar with the role of the retinoblastoma gene in ocular cancer. However, disruption of the retinoblastoma tumor suppressor pathway has been shown in virtually all cancers, including uveal melanoma. Inactivation of the retinoblastoma protein by hyperphosphylation leads to unregulated cell proliferation. In uveal melanoma, insight into subsequent cellular events is coming into focus. It is here that further evaluations may yield treatment advances. Several mechanisms exist to eliminate damaged cells. Drugs aimed at these mechanisms may permit treatment of primary and metastatic disease. Moreover, insights into events that may contribute to the development of metastatic potential are being elucidated.
The discovery of oncogenes, and tumor suppressor genes, has led to new insights into tumor development and novel treatments. Malignant tumors typically contain multiple mutant genes. If we look at other cancers, the development of malignancy appears to occur gradually over time with a series of steps preceding malignant transformation. Cancers such as cervical, colon and lung, all can be traced through early stages of premalignant, noninvasive cells, prior to malignant and invasive cell emergence. While melanoma can develop de novo, most are believed to develop from preexisting nevi. Nevi that grow may have developed some mutations that permit limited growth, but not malignant transformation and invasion. Further mutational events may permit malignant transformation, but not invasive qualities. Other mutational events may confer on some melanomas the ability for invasion and metastasis. These melanomas would correspond to the Class 2-type tumors. Improved understanding of these mutational events, and the mechanism of action of these genes will provide the potential for new treatments, as we have seen in other areas of oncology. Ocular oncology appears to be on the verge of achieving significant insights into melanoma cell biology. With this progress, we can hopefully achieve a significant improvement in survival from this potentially deadly disease.
Dr. Johnson is an attending at CPMC, an adjunct assistant clinical professor at the University of California San Francisco, and in private practice at the West Coast Retina Medical Group. Contact him at West Coast Retina Medical Group, Lobby 2, Ste. 130, 185 Berry St., San Francisco, Calif. 94107. Phone: (415) 972 4600; fax: (415) 975 0999; e-mail:
bj.johnson@comcast.net.
1. Ferry, AP. Lesions mistaken for malignant melanoma of the posterior uvea. Arch Ophthalmol 1964;72:463-469.
2. Shields, JA and Zimmerman, LE. Lesions simulating malignant melanoma of the posterior uvea. Arch Ophthalmol 1973;89:446-471.
3. Histopathologic characteristics of uveal melanomas in eyes enucleated from the Collaborative Ocular Melanoma Study. COMS report no. 6. Am J Ophthalmol 1998;125:745-66.
4. Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol 2006;124:1684-93.
5. Hawkins, BS and Collaborative Ocular Melanoma Study Group.The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol 2004;138:936-51.
6. Collaborative Ocular Melanoma Study Group. Trends in Size and Treatment of Recently Diagnosed Choroidal Melanoma, 1987-1997. Findings from patients examined at Collaborative Ocular Melanoma Study (COMS) centers: COMS Report No. 20. Arch Ophthalmol 2003;121:1156-1162.



