Traditional Local Treatment
In most cases, the initial treatment for NIU-PS is corticosteroids. For uveitis, these may be administered locally or systemically. Current local options for corticosteroid administration include ophthalmic drops, local injection (sub-Tenon’s or intravitreal) and implants (the dexamethasone intravitreal implant [Ozurdex, Allergan] and the fluocinolone acetonide intravitreal implant [Retisert, Bausch + Lomb]). In addition to risks from the individual form of administration (such as globe perforation for sub-Tenon’s injection and endophthalmitis for intravitreal injection), side effects of local ophthalmic corticosteroids include a high rate of cataract and complications due to increased intraocular pressure.4
New Delivery Systems
Currently, there are two studies looking at novel delivery approaches for corticosteroids to treat non-infectious uveitis. The PEACHTREE (Clearside Biomedical) study is a Phase III, randomized, masked, multicenter, controlled clinical trial to study the safety and efficacy of a triamcinolone acetonide suspension injected into the suprachoroidal space of subjects with macular edema associated with non-infectious uveitis. This study is designed to evaluate whether injections every 12 weeks may provide control of uveitic macular edema with fewer side effects than sub-Tenon’s or intravitreal steroid injections.
Iontophoresis is the use of an electric current to help propel molecules across the hydrophilic cornea epithelium, classically the largest barrier to drug penetration into the eye. This would theoretically increase the bioavailability of the steroid medication, allowing greatly reduced dosing frequency and increased efficacy as compared to topical corticosteroids. This method has the advantage of not being associated with many of the needle-related risks of other local treatments. EyeGate Pharmaceuticals is currently examining iontophoresis for the delivery of dexamethasone into the eye to treat anterior non-infectious uveitis.
Intravitreal Sirolimus
Sirolimus is an immunomodulating agent that works via the mTOR pathway, reducing the number of active T-cells and the amount of inflammatory cytokines. Oral sirolimus has been shown to be effective for treating non-infectious posterior uveitis, but its side-effect profile and need for laboratory monitoring make it a less desirable treatment choice.5 A hydrophobic intravitreal formulation of sirolimus, which forms a depot lasting approximately two months in the vitreous (See Figure 1) has been evaluated in the SAKURA (Sirolimus study Assessing double-masKed Uveitis tReAtment) I and II studies (Santen Pharmaceuticals). In December of last year, the FDA informed Santen that its intravitreal sirolimus application wasn’t approvable in its current form, and the agency would need additional evidence of efficacy.
The SAKURA I results were published in 2016. This study examined 347 patients who received 44, 440 or 880 micrograms of sirolimus intravitreally. Patients were required to be off of all systemic immunomodulatory medications, except for oral steroids, which were rapidly tapered at the start of the study per protocol. The main study outcome was the proportion of patients with no vitreous haze at the end
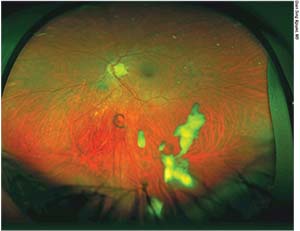 |
| Figure 1. An intravitreal depot of sirolimus 14 days after injection. |
What is potentially exciting about intravitreal sirolimus is the lower rate of ocular side effects compared to intravitreal corticosteroids. In the study, there was a low rate of cataract and IOP change. The most common ocular adverse events were inflammatory, and there were some instances of opaque drug depot in the visual axis. There were no systemic serious adverse events related to the drug.
Systemic Treatments
Oral prednisone is very effective for the treatment and prevention of recurrence in NIU-PS. In addition to the ophthalmic side effects mentioned above, however, oral prednisone can lead to a multitude of systemic side effects, including hyperglycemia, dyslipidemia, hypertension; osteoporosis and fractures; immunosuppression and gastric reflux.
In addition to corticosteroids, other traditional options for systemic treatment include antimetabolites, T-cell inhibitors and alkylating agents. The antimetabolites include methotrexate and mycophenolate mofetil and tend to be the first-line agents for steroid-sparing systemic treatments due to their relatively favorable side-effect profile. Cyclosporine and tacrolimus are T-cell inhibitors that that can be associated with hypertension and nephrotoxicity. Cyclophosphamide and chlorambucil are alkylating agents that are used for uveitis associated with severe systemic disease, such as granulomatosis with polyangitis. Their side effects include pancytopenia, hemorrhagic cystitis and malignancies.
The Multicenter Uveitis Steroid Treatment (MUST) trial compared systemic anti-inflammatory therapy to sustained-release corticosteroid implants for NIU-PS. This longitudinal study showed that although local therapy led to faster control of inflammation, after seven years the systemic medication group had better visual acuity outcomes. The implant therapy eyes had statistically significantly higher rates of complications related to IOP and cataracts, while the systemic therapy group had a significantly higher rate of infections requiring treatment.4,7
New Systemic Options
Adalimumab (Humira, AbbVie) is a tumor necrosis factor alpha inhibitor given by subcutaneous injection. It has been used for more than a decade for inflammatory conditions such as rheumatoid arthritis, psoriasis and Crohn’s disease. The VISUAL I and II trials showed benefit of adalimumab for patients with active and inactive NIU-PS, respectively, requiring systemic prednisone.8,9 Patients receiving adalimumab (80 mg loading dose followed by 40 mg every other week) were half as likely to fail steroid taper than those receiving placebo. Use of adalimumab was also associated with an approximately doubled time to treatment failure as compared to placebo. Based on VISUAL I and II, in 2016 the FDA expanded the indications of adalimumab to include NIU-PS.
Other Biologics
The STOP-UVEITIS study is currently evaluating tocilizumab (an anti-interleukin-6 antibody) for patients with non-infectious uveitis. This study looks at monthly IV infusions of tocilizumab in two doses and is scheduled to conclude in December of 2017 (https://clinicaltrials.gov/ct2/show/NCT01717170). Secukinumab is an anti-interleukin 17A antibody that was shown to be effective in quieting posterior uveitis and improving remission rates.10 Intravenous treatment was more effective than subcutaneous treatment. Sarilumab is another anti-IL-6 antibody that resulted in decreased vitreous haze and decreased oral steroid dosing when given subcutaneously every two weeks.11
In conclusion, though NIU-PS continues to be a challenging condition to treat, there continue to be more therapeutic options. For local treatment, intravitreal Sirolimus may become available soon and, systemically, adalimumab is a newly approved FDA option. Also, there are many therapeutics in the pipeline that offer novel agents and new ways to use traditional drugs.
Dr. Atchison is a fellow at Illinois Retina Associates and Rush University, Chicago. Dr. Merrill is a partner at Illinois Retina Associates and an assistant professor in ophthalmology at Rush. Dr. Atchison may be reached at elizabethatchison@gmail.com. Dr. Merrill may be contacted at pauline.merrill@gmail.com. They’d like to thank Quan Dong Nguyen, MD, for his contributions to the article.
1. Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: Results from the pacific ocular inflammation study. JAMA Ophthalmol 2013;131:11:1405-12.
2. Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology 2016;123:3:655-62.
3. Frick KD, Drye LT, Kempen JH, et al. Associations among visual acuity and vision- and health-related quality of life among patients in the multicenter uveitis steroid treatment trial. Invest Ophthalmol Vis Sci 2012;53:3:1169-76.
4. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group, Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: The multicenter uveitis steroid treatment trial. Ophthalmology 2011;118:10:1916-26.
5. Shanmuganathan VA, Casely EM, Raj D, et al. The efficacy of sirolimus in the treatment of patients with refractory uveitis. Br J Ophthalmol 2005;89:6:666-9.
6. Nguyen QD, Merrill PT, Clark WL, et al. Intravitreal sirolimus for noninfectious uveitis: A phase III sirolimus study assessing double-masKed uveitis TReAtment (SAKURA). Ophthalmology 2016;123:11:2413-23.
7. Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group, Kempen JH, Altaweel MM, et al. Association between long-lasting intravitreous fluocinolone acetonide implant vs. systemic anti-inflammatory therapy and visual acuity at 7 years among patients with intermediate, posterior, or panuveitis. JAMA 2017;317:19:1993-2005.
8. Jaffe GJ, Dick AD, Brezin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:10:932-43.
9. Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): A multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:10050:1183-92.
10. Letko E, Yeh S, Foster CS, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology 2015;122:5:939-48.
11. de Smet m, Sundaram P, Erickson K, et al. Variability in vitreous haze grading technique using a 9-step logarithmic scale in patients enrolled in the sarilumab in noninfectious uveitis (SARIL-NIU), the SATURN study. In ARVO Proceedings; 2016.



