Kenneth J. Mandell, MD, PhD, Amanda J. Howe,
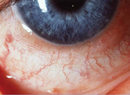
Allergic conjunctivitis affects upwards of 20 percent of the
Anatomy and Physiology of the Conjunctiva
The conjunctiva is anatomically divided into two layers: the epithelium and substantia propria.1 The epithelium is continuous with the skin at the mucocutaneous junction on the lid and the corneal epithelium at the limbus. It is stratified, with two to seven layers of epithelial cells, as well as goblet cells, melanocytes, Langerhans cells and inflammatory cells intercalated within them. The epithelial cells are joined by desmosomes and tight junctions, which serve to regulate adhesion. Subjacent to the conjunctival epithelium is the substantia propria, which contains mast cells, plasma cells, lymphocytes and neutrophils, all of which increase in number in allergic inflammation.1
The conjunctiva is the most immunologically active tissue of the ocular surface, as it is exposed to countless pollens, danders and other proteins, as well as irritants and pollutants.1 The eyelids and tear film provide the first line of defense through the pumping action of the blink and tear lavage, but the conjunctiva itself also has several defense mechanisms. Conjunctival goblet cells produce mucins, which have anti-microbial activity. The conjunctiva also produces other natural anti-infective peptides, such as lactoferrin. Also, conjunctival epithelial cells act as a mechanical barrier preventing influx of microorganisms, toxins and the like remaining on the ocular surface.1
Together these structures and soluble mediators form a physical and biochemical barrier to ocular surface penetration.
The Epithelial Barrier
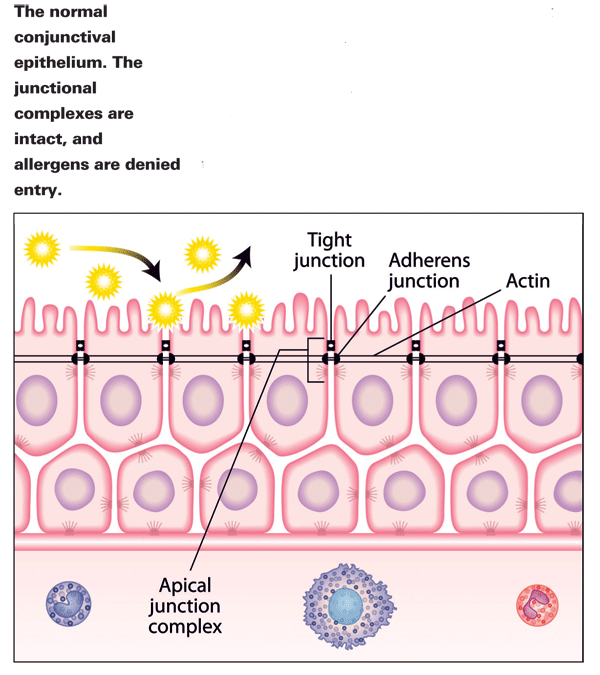
The conjunctival surface is a complex and dynamic structure that plays a key role in the prevention of inflammatory disease. An extensive network of epithelial cells is linked together to form the conjunctival epithelial barrier. At the basement membrane, hemidesmosomes are present to anchor the epithelial cells. Inflammatory cells reside beneath the basement membrane; mast cells, eosinophils and other substances migrate along the concentration gradient in this region until allergens break through the epithelial barrier, are taken up by antigen presenting cells (APCs), cross-link with IgE and set off a cascade of allergic mediators that drive the inflammatory cells up through the paracellular route of the epithelial barrier to the cell surface. This results in redness, itching and inflammation. It can, however, be stopped. Cell-cell attachment of the epithelial barrier is mediated by desmosomes near the basement and tight-junction complexes near the apex, as well as gap junctions in between. The TJ complexes (consisting of zonula occludens, zonula adherens and E-cadherins) form a seal between epithelial cells, and under normal circumstances only grant non-adversarial molecules passage. Aside from cell-cell connection, epithelial cells interact with leukocytes via adhesion molecules expressed on their surface. Mucosal immune cells (Langerhans cells, APCs and other dendritic cells) play an active, participatory role in immune reactions through their expression of surface antigens and the synthesis of cytokines.2
Tight Junctions in Allergic Disease
Contiguous expression of junction complexes along the apical perimeter of cells forms a sealant ring, intended to deny foreign proteins access to paracellular routes.3 This seal can be weakened, however, by a variety of "openers," or molecules and proteins that unlock the elastic junctional complex. Allergens, irritants and pollutants do this to varying degrees and can make lasting changes to the epithelium. By doing so, they also facilitate easier entry for their counterparts at a later time. Poly-aromatic hydrocarbons, which are major pollutants and key factors in urban allergy, have been shown to open the barrier, as have pan-seasonal allergens.3 People living in cities have higher rates of allergy than their rural counterparts,4 and pollutants can damage the cell walls of pollen grains to the extent that their proteinaceous contents are released.5
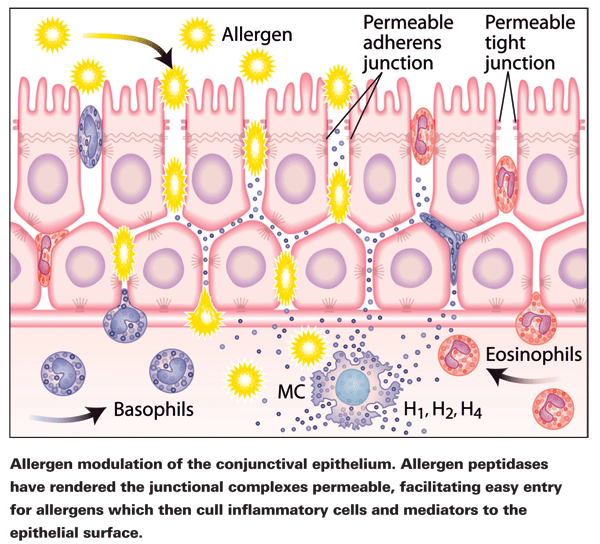
Following exposure to house dust mite fecal particles, immunostaining has shown reduced TJ continuity as well as reductions in the levels of ZO-1 and occludin staining, presumably due to TJ fragmentation.3 Interestingly, atopic patients, even out of season, have significantly altered epithelia compared to non-atopic patients, although, clinically, the conjunctiva appears normal as evidenced by E-cadherin expression.6 The epithelial barrier should be a blocking point in the development of allergy, but the ability of allergens to modulate it renders it ineffective.
Modulation of the Epithelial Barrier
Pollens carry proteolytic enzymes on their surface that are believed to be involved in the fertilization process.7 It's likely that these properties evolved to degrade pectin in plant cell walls. In the setting of allergy, these same enzymes degrade components of epithelial junctions (occludin, claudins and ZO-1) as well. Diffusates from giant ragweed (GRW), white birch (WB), Kentucky bluegrass (KBG) and Easter lily (EL) pollen were used in a study of pollen's effect on TJs. When treated with KBG, GRW, WB or EL pollen, TJ staining showed breaks in the contiguous rings at all time points, and some cell interfaces were devoid of staining at eight hours.7
In a different study, the presence of an active allergic reaction in seasonal allergic conjunctivitis patients was associated with a significant thickening of the conjunctival epithelium. Out of season, thickening was not statistically significantly different.6
Immunohistochemical analysis also showed that the areas of staining for E-cadherin and CD44 (a TJ marker) out of season were significantly lower than those observed in normal patients.6 Compared with SAC patients out of season, those with an active reaction had statistically significantly greater expression of E-cadherin and CD44, though not statistically different from controls. These findings suggest that SAC patients out of season have structurally weaker epithelia, which contribute to increased susceptibility to allergen penetration.6 This is then followed by an influx of inflammatory cells that disrupt the epithelial barrier via release of inflammatory mediators.6
Investigators have also compared various inflammatory markers in normal patients and those with vernal keratoconjunctivitis and atopic keratoconjunctivitis through immunohistochemical staining.2 They found that IL-3, GM-CSF, ICAM-1 and HLA-DR were not expressed in normal conjunctival epithelial cells. However, patients with VKC and AKC had elevated levels of the various markers. They also examined epithelial junction susceptibility to degradation by human mast cell chymase and found that occludin and fibronectin were cleaved by chymase, inhibiting epithelial cell migration.2
Similarly, eosinophil cationic proteins such as MBP and ECP in tear fluid have demonstrated a cytotoxic effect on corneal epithelial cells in vitro.8 And mast cell mediators including platelet-activating factor, histamine, IL-4, tumor necrosis factor-a, and interferony have also been shown to modulate epithelial function in vitro.8
Furthermore, changes in phosphorylation of myosin light chains have been shown to affect barrier integrity of corneal epithelial cells by histamine-induced mechanisms.9
The Effect of Perennial Allergens
House dust mites and cockroaches are the allergens most commonly associated with atopic manifestations like asthma, rhinitis and atopic dermatitis.10,11 They are ubiquitous worldwide, but the largest populations reside in more humid areas. Greater indoor humidity is therefore associated with increased numbers. House mites live primarily in bedding, upholstered furniture and carpeting, but especially enjoy down pillows and comforters.11 Furthermore, the type of heating, number of occupants, ratio of carpeting to hard floors and the age of a home can be determining factors in dust mite habitation.11
House dust mite fecal pellets contain proteolytic enzymes that have been shown to disrupt tight junctions.3 These allow allergens to be delivered to APCs, similar to the action of pollen peptidases. When inhaled, the fecal pellets discharge their contents onto the respiratory epithelium. They can also do so upon landing on the "collecting plate" of the ocular surface.
Der p 1, the most extensively studied dust mite fecal-pellet protein, is similar to the plant enzyme papain, implicated in allergy.3 Der p 1 cleaves occludin and claudin, CD23 and CD25; attacks the alpha subunit of CD25; degrades the alpha-1 proteinase inhibitor; and skews murine T-cells to Th2 response. Der p 9 (another fecal-pellet protein) has been shown to increase the release of GM-CSF, IL-6 and IL-8.3
There is a wealth of evidence supporting epithelial barriers as key players in the allergic cascade. Prior to pollen or pollutant modulation, they are strong inhibitors of transepithelial flux by adversarial agents. However, pollens disrupt barrier proteins and gain access to APCs, initiating allergen cross-linking and mast cell degranulation. More scientific investigation of the conjunctival epithelium is warranted, as well as efforts to identify novel therapies that may be able to act upstream of mast cell stabilizers/antihistamines.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Irkec M, Bozkurt B. Epithelial cells in ocular allergy. Curr Allergy Asthma Rep 2003;3:4:352-7.
2. Hingorani M, Calder VL, Buckley RJ, Lightman SL. The role of conjunctival epithelial cells in chronic ocular allergic disease. Exp Eye Res 1998;67:5:491-500.
3. Wan H, Winton HL, Soeller C, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy 2001;31:2:279-94.
4. Priftis KN,
5. Chehregani A, Majde A, Moin M, et al. Increasing allergy potency of Zinnia pollen grains in polluted areas. Ecotoxicol Environ Saf 2004;58:2:267-72.
6. Hughes JL, Lackie PM, Wilson SJ, et al. Reduced structural proteins in the conjunctival epithelium in allergic eye disease. Allergy 2006;61:11:1268-74.
7. Runswick S, Mitchell T, Davies P, et al. Pollen proteolytic enzymes degrade tight junctions. Respirology 2007;12:6:834-42.
8. Ebihara N, Funaki T, Murakami A, et al. Mast cell chymase decreases the barrier function and inhibits the migration of corneal epithelial cells. Curr Eye Res 2005;30:12:1061-9.
9. Guo Y, Ramachandran C, Satpathy M, Srinivas SP. Histamine-induced myosin light chain phosphorylation breaks down the barrier integrity of cultured corneal epithelial cells. Pharm Res 2007;24:10:1824-33.
10. Huss K,
11. Chan-Yeung M, Becker A, Lam J, et al. House dust mite allergen levels in two cities in
12. Miraglia Del Giudice M, Pedulla M, Piacentini GL, et al. Atopy and house dust mite sensitization as risk factors for asthma in children. Allergy 2002;57:2:169-72.
13. Roelandt T, Heughebaert C, Hachem JP. Proteolytically active allergens cause barrier breakdown. J Invest Dermatol 2008;128:8:1878-80.