 |
|
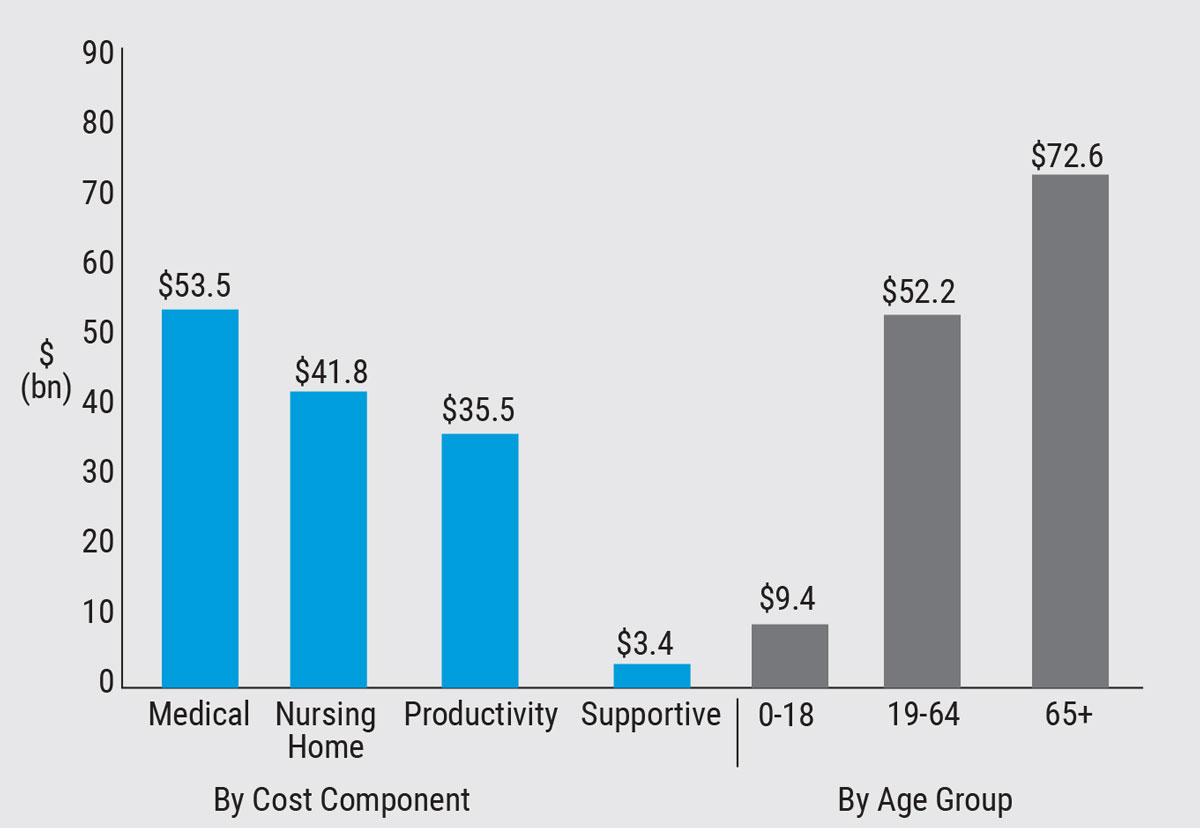
This graph shows the total cost of vision loss or blindness by burden component and age group in billions of dollars. (Source: Rein DB et al. 2022.) |
Vision loss and blindness pose a significant economic burden on the United States. Public health researchers at the National Opinion Research Center (NORC) at the University of Chicago confirmed the substantial economic hardship in a recently published cost estimate for the year 2017 and indicated that vision-loss resource allocation will likely differ by state due to the varied composition of per-person costs.
In the study, which was supported by a research contract from the Centers for Disease Control and -Prevention’s Vision Health Initiative, the researchers analyzed secondary data sources including the American Community Survey, American Time Use Survey, Bureau of Labor Statistics, Medical Expenditure Panel Survey, National and State Health Expenditure Accounts and National Health Interview Survey. Study participants included those who answered “yes” to the question, “Are you blind or do you have serious difficulty seeing even with glasses?”
“Based on that, we estimated that the total burden was $134.2 billion,” says David B. Rein, PhD, a program area director overseeing public health analytics at NORC’s Public Health department. “Of this, most of the costs were direct costs totaling $98.7 bn and indirect costs totaling $35.5 bn. The largest components of total costs were incremental nursing home costs ($41.8 bn), other medical costs including glasses and contact lenses and home health care ($30.9 bn) and reduced labor force participation ($16.2 bn).”
The total costs per person were fairly high at $16,840 per affected person per year. “The sources of these costs varied by age group,” he says. “For children 18 and younger, the main costs were associated with informal care provided by parents or caregivers who were spending extra time caring for children. For adults ages 19 to 64, the main costs came from reduced labor force participation, and for older adults (65 and older) the main costs were increased placement in nursing homes.
“State variations in costs were driven by regional differences in health care and nursing home costs, and by the age distribution of the state’s population,” Dr. Rein continues. “New York, Connecticut, Massachusetts, Rhode Island and Vermont had the highest costs per person with vision loss. The highest and lowest total burdens were seen in California ($13.5 bn) and Wyoming ($191 million), respectively.” For more information on the cost breakdown by state and category, visit cdc.gov/visionhealth/economics.
“We did our best to create this estimate, but there’s a lot of variability and uncertainty,” he notes. “We feel good about the choices we made in estimating the burden, but it’s plausible that the true burden could be as low as $76 bn or as high as $218 bn, based on different structural assumptions in the model, which we explain in the paper. This study measured costs only among people who report they are blind or have severe difficulty seeing even when wearing glasses. This likely underestimates the number of people with any type of vison loss in the country. We only measured costs associated with vision loss and blindness to get a clearer picture of the vision loss burden; we didn’t estimate the costs for the care of all eye diseases that haven’t resulted in vision loss, or the costs of routine eye care.”
Dr. Rein notes two major differences between this study and previous NORC estimates from 2006 and 2013. “In the present study, we looked only at costs associated with vision loss and blindness, and we didn’t attempt to estimate any medical care costs for vision or eye conditions that hadn’t yet resulted in vison loss or blindness,” he explains. “Additionally, we expanded the definition of vision loss to include all those who reported they were blind or had serious difficulty seeing, even with glasses, regardless of whether that vision impairment is correctable. Uncorrected refractive error is a large source of vision impairment in the United States and ignoring its impact will lead to underestimation of the true burden.
“It’s difficult to make an exact comparison among studies,” he continues, “but it seems we’re estimating higher costs than we did in the past—certainly compared to our 2006 estimate, which was limited primarily to medical care costs associated with four major vision and eye conditions. What we’ve shown in the present study is that there’s a substantial cost associated with vision impairment and blindness itself.”
Dr. Rein says prevention is key. “The policy change that would have the biggest bang for the buck would be increased efforts to identify and treat uncorrected refractive error,” he says. “There are large potential savings in this—not just for medical care but in potentially averting productivity losses and reduced informal care—and of course the intangible but equally important benefits of improving people’s day-to-day vision. Ensuring routine eye exams and eye health services are available and accessible to everyone regardless of income or insurance status or where they live would be another policy change that, while incurring costs upfront, will potentially save costs in the long run.”
How might the COVID-19 pandemic have impacted these 2017 cost estimates? “We’re all waiting on the data to see what happened with COVID,” says Dr. Rein. “I think one major change would be the prevalence of vison loss in different populations. There’s evidence that at least some people avoided necessary care during the pandemic who would otherwise have gotten it. Some of this avoidance may have resulted in irreversible vision loss.
“There’s also some evidence that severe SARS-CoV-2 infections can result in vision problems, and there are other vision problems that may be associated with long COVID,” he says. “That would increase the number of individuals reporting vision problems in the years to come, and costs would be higher. On the other hand, COVID also hit the elderly population with the highest rates of mortality, especially among those in nursing homes, so the overall burden might decrease.
“Increased screen time associated with the pandemic may also be contributing to increases in myopia, especially among children,” he adds. “However, we need to wait for additional surveys, claims datasets and EHR records that can give us better data on these questions.”
Ultimately, Dr. Rein says this study helps to quantify the magnitude of the problem of vision loss and blindness in the United States. “The economic burden of vision loss is greater than many other conditions that also often get more attention,” he says. “Ophthalmologists and optometrists can dramatically improve their patients’ quality of life. Our study shows that eye care can also have the potential to reduce the economic burden of vision loss on society as a whole.”
Rein DB, Wittenborn JS, Zhang Ping, et al. The economic burden of vision loss and blindness in the United States. Ophthalmology 2022;129:369-78.
Industry Briefs First Installment of The Video Journal of Cataract, Refractive & Glaucoma Surgery Now Available The Video Journal is a free member benefit of virtually every cataract society, and a new one is released quarterly. To view the lectures free of charge, visit www.vjcrgs.com.
Allergan Announces Topline Results for New Vuity Regimen |
Risk Factors for Tube-shunt Revisions
Glaucoma drainage devices reduce intraocular pressure by creating an alternate drainage route for aqueous humor to bypass the diseased trabecular meshwork. Despite their benefits, GDDs are not without complications, such as tube exposures, that may necessitate repeat surgery. Researchers at Massachusetts Eye and Ear were able to elucidate subtle associations between both demographic and clinical characteristics and GDD removal or revision surgery by using data from the IRIS Registry. Dry-eye disease and chronic angle-closure glaucoma were associated with a higher risk of GDD revision or removal surgery, while factors such as diabetes, history of smoking and unknown/unreported race and ethnicity were associated with a lower risk of repeat GDD surgery.
The Registry noted 44,330 distinct patients who underwent at least one GDD implantation, 7.6 percent of whom underwent subsequent GDD revision or removal surgery within six years. This incidence was lower than previously reported rates. Stratified risk analyses demonstrated that unknown race/ethnicity (HR: 0.83/0.68), diabetes (HR: 0.84) and history of smoking (HR: 0.86) decreased the risk of GDD revision, while chronic angle-closure glaucoma (HR: 1.32) and dry-eye disease (HR: 1.30) were associated with increased rates of GDD revision. Asian and black patients were noted to have a decreased risk of GDD removal, while Hispanic patients were at increased risk for GDD revision.
Factors associated with a decreased average time (in days) from original GDD surgery to revision/removal included male sex, unknown race and right-eye laterality. Factors associated with an increased average time to GDD revision/removal included a history of a past eye procedure and active smoker status. Patients with diabetes were found to have GDD revision/removal earlier during their follow-up period compared with patients without diabetes.
“Although current evidence on risk factors associated with GDD revision or removal surgery is conflicting, the power provided by the enormity and diversity of the IRIS Registry may provide a more accurate representation of the factors associated with repeat GDD surgery,” the authors concluded in their paper. “Thus, it is helpful to consider the aforementioned factors when determining the prognosis of GDD surgery and choice for treatment.”
Hall NE, Chang EK, Samuel S, et al. Risk factors for glaucoma drainage device revision or removal using the IRIS Registry. Am J Ophthalmol. April 2, 2022. [Epub ahead of print].



