Though children under 13 are banned from using most products involving virtual reality headsets, researchers at St. Louis Children’s Hospital say that might not be necessary.
In the prospective, interventional, before-and-after study, the investigators studied 50 children (29 boys) as they played a VR flying game on the Sony Playstation 4. The children used their head movements to control their flight in the game. Baseline testing preceded VR exposure, and each VR session was followed by testing of binocular corrected distance visual acuity, refractive error, binocular eye alignment, stereoacuity and postural stability. Visually induced motion sickness was probed using the Simulator Sickness Questionnaire modified for pediatric use (Peds SSQ). Visual-vestibulo-ocular reflex (V-VOR) adaptation was also tested pre- vs. post-trial in five of the children.
Surprisingly, the researchers found that VR discomfort in kids may be even less than that reported in adults. Forty-six of the 50 children (94 percent) completed both VR play sessions with no significant change from baseline in measures of binocular CDVA (p=0.89), refractive error (p=0.36), binocular eye alignment (p=0.90), or stereoacuity (p=0.45). Postural stability degraded an average of 9 percent from baseline after 60 minutes of VR exposure (p=0.06). Pre- versus post-trial, the Peds SSQ scores increased a mean 4.7 percent for each of four symptom categories: eye discomfort (p=0.02); head/neck discomfort (p=0.03); fatigue (p=0.03); and motion sickness (p=0.01). None of the children who finished both trial sessions (94 percent) asked to end the play time. V-VOR gain remained unaltered in the five children tested. Three children (6 percent)-—two girls (ages 5 and 6 years) and one boy (age 7 years)—discontinued the trial during the first 10 minutes of the first session. The girls reported discomfort consistent with mild motion sickness, while the boy said he was bored and the headset was uncomfortable. No child manifested aftereffects in the days following the VR exposure.
Am J Ophthalmol 2020; 209:151-159
Tychsen L, Foeller P.
Leakage on FA After Intravitreal Aflibercept Injections
Researchers aimed to characterize leakage indices on ultra-widefield fluorescein angiography in proliferative diabetic retinopathy treated with intravitreal aflibercept. The prospective study enrolled subjects for treatment of proliferative diabetic retinopathy randomized 1:1 to receive 2-mg of intravitreal aflibercept every four weeks (2q4) or every 12 weeks (2q12).
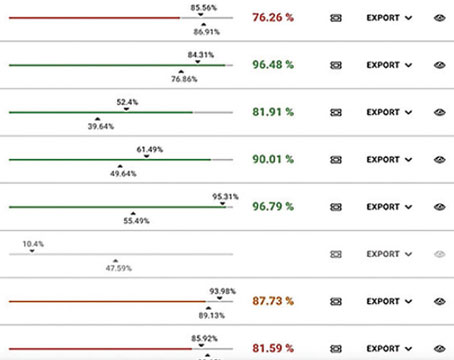
Researchers analyzed ultra-widefield FA images obtained at baseline, 24 and 48 weeks using a semiautomated leakage segmentation platform. Forty eyes of 40 subjects were included, and mean age was 48 ±12.1 years. Mean number of injections was 11 ±1.7 in the 2q4 arm and 4 ±0.4 in the 2q12 arm. Median baseline leakage index in the 2q4 arm was 5.1 percent, and median baseline leakage index in the 2q12 group was 4.3 percent (p=0.28). Researchers found:
• At 24 and 48 weeks, the 2q4 group significantly improved to 1.1 percent (-79 percent, p<0.0001).
• At week 24, the 2q12 group demonstrated non-significant improvement (3.4 percent; -21 percent, p=0.47); by week 48, improvement was significant (1.4 percent; -68 percent, p=0.02).
• The 2q4 group had a lower leakage index (1.1 percent) compared with the 2q12 group at 24 weeks (3.4 percent)(p=0.008); but by 48 weeks, the leakage index was similar between both groups (1.1 percent [2q4 group] vs. 1.4 percent [2q12 group]; p=0.34).
Researchers found that PDR treated with intravitreal aflibercept demonstrated significant leakage index reductions at one year. Monthly dosing provided more rapid reductions in leakage index than quarterly dosing. REVIEW
Retina 2020; Jan 8. [Epub ahead of print].
Babiuch AS, Wykoff CC, Srivastava SK, et al.