Ranibizumab
In April 2017, ranibizumab was approved by the FDA for treatment of all forms of diabetic retinopathy. Before that point, however, several studies began showing the drug’s treatment potential.
RISE and RIDE were large multicenter trials evaluating the use of ranibizumab (Lucentis, Genentech; South San Francisco, Calif.) for DME.1 Patients were randomized to receive 0.3-mg or 0.5-mg ranibizumab or sham injections. In addition to showing ranibizumab’s efficacy for treatment of DME, a secondary analysis showed a ≥3-step improvement in Diabetic Retinopathy Severity
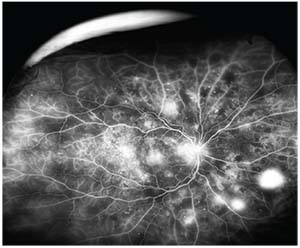 |
| Figure 1. Fluorescein angiography showing areas of retinal neovascularization consistent with proliferative diabetic retinopathy. |
An open-label extension of RISE and RIDE enrolled 500 patients who had completed the original 36-month randomized studies.2 Patients were offered ranibizumab 0.5 mg as needed based on predefined criteria, and outcomes at 48 months were examined. At 48 months, 11.2 percent and 7.6 percent of patients in the 0.3-mg and 0.5-mg groups achieved a ≥3-step DRSS improvement, respectively, compared to 4.8 percent of patients in the sham-with-crossover group. Only 2.5 percent of patients receiving ranibizumab had a ≥2-step worsening, compared to 11.3 percent of sham patients. Patients originally randomized to ranibizumab had an overall lower risk of PDR development than the sham group, and this finding persisted to month 54.2
Diabetic Retinopathy Clinical Research Protocol I was a randomized trial comparing focal laser (L), triamcinolone plus focal laser (T+L), ranibizumab plus prompt focal laser (R+pL), and ranibizumab plus deferred laser (R+dL) for DME.3 An additional analysis was performed to evaluate the effects of intravitreal ranibizumab and triamcinolone on worsening of diabetic retinopathy in this study.4 For patients without PDR, at 36 months, retinopathy worsened in: 7 percent in R+dL; 18 percent in R+pL; 23 percent in sham+L; and 37 percent in T+L.
Among patients with PDR, at 36 months, 18 percent, 21 percent, 40 percent and 12 percent had worsening of retinopathy among the same respective groups. Patients who received ranibizumab or triamcinolone had lower rates of vitreous hemorrhage and were less likely to require panretinal photocoagulation laser treatment.
DRCR Protocol S compared ranibizumab to PRP for high risk PDR.5 Patients with PDR were randomized to six ranibizumab injections every four weeks followed by as-needed retreatment at follow-up or PRP. The study demonstrated that ranibizumab treatment was non-inferior to PRP with regard to visual acuity outcomes. Additionally, patients with PDR treated with ranibizumab progressed less than patients treated with PRP, had less-affected peripheral vision, had more improvement in terms of central macular thickness and were less likely to go on to vitrectomy than patients who received PRP.5
Aflibercept
Aflibercept is FDA-approved for diabetic retinopathy in the setting of DME. A discussion of the following major studies explains its potential treatment benefit.
VIVID and VISTA were large multicenter studies comparing intravitreal aflibercept (Eylea, Regeneron; Tarrytown, N.Y.) to focal laser for the treatment of DME.6 Patients were randomized to receive focal laser, 2 mg of intravitreal aflibercept every four weeks or 2 mg of intravitreal aflibercept every eight weeks after five monthly loading doses. Patients who received aflibercept had better visual acuity improvement than those who received focal laser, and also were more likely to have ≥2-step improvement in DRSS score. Patients in both aflibercept groups were three times more likely to achieve ≥2-step DRSS improvement than patients in the laser group.
DRCR Protocol T was a randomized clinical trial comparing aflibercept 2 mg, bevacizumab 1.25 mg and ranibizumab 0.3 mg for the treatment of DME.7 A secondary analysis was performed to evaluate the effects on DRSS with these agents.8 At one year, in patients with NPDR, improvement in diabetic retinopathy severity was seen in 31.2 percent with aflibercept, 22.1 percent with bevacizumab and 37.7 percent with ranibizumab. In patients with PDR, improvement in diabetic retinopathy severity was seen in 75.9 percent for aflibercept, 31.4 percent for bevacizumab and 55.2 percent for ranibizumab. These effects persisted at two years. These findings demonstrate less improvement with bevacizumab than with the other two agents in terms of DRSS, and show that aflibercept was associated with more improvement in patients with PDR.
Bevacizumab
The intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT) study compared bevacizumab (Avastin, Genentech; South San Francisco, Calif.) to focal laser for DME.9 Patients in the bevacizumab group had significant BCVA i
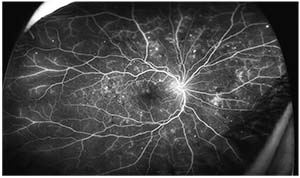 |
| Figure 2. Fluorescein angiography of the same eye shown in Figure 1, following intravitreal bevacizumab injections, demonstrating regression of neovascularization. |
As mentioned above, DRCR Protocol T compared bevacizumab with ranibizumab and aflibercept for diabetic macular edema, and found that all three agents reduce DR severity through two years of follow-up, although patients treated with bevacizumab had less improvement than aflibercept or ranibizumab.8
Bevacizumab is frequently used off-label for the treatment of DME and is a cost-effective alternative to ranibizumab or aflibercept. Because bevacizumab blocks VEGF, it likely will improve diabetic retinopathy. However, further investigation is needed to fully evaluate the effects of bevacizumab on DR.
Discussion
Secondary analyses of several large clinical trials have demonstrated the benefit of anti-VEGF treatment, specifically ranibizumab and aflibercept, at halting—and in some cases reversing—DR. The clinical importance of these improvements in DRSS score with the administration of intravitreal anti-VEGF was evaluated by the Doheny Eye Institute’s Michael Ip, MD, and his colleagues.10 Based on a post-hoc analysis of data from RISE and RIDE, BCVA improvement of ≥15 letters was 51.9 percent and 44.6 percent in patients who had a two- or three-step improvement in DRSS, respectively. In contrast, ≥15 letter improvement in patients with either a one-step DRSS improvement, no change, or worsening of DRSS, was 37.9 percent, 39.6 percent and 26.7 percent, respectively. In addition, a loss of ≥15 letters was seen in 13.3 percent of patients with DRSS worsening compared to zero to 2.8 percent in patients with stable or improved DRSS. Patients with DRSS improvement were also more likely to achieve resolution of macular edema than those with stable or worsening DRSS. Thus, improvements in DRSS with ranibizumab treatment were also associated with better vision and better DME response to treatment. Lower rates of transitioning from NPDR to PDR mean less vitreous hemorrhage and less need for PRP.
Protocol S demonstrated noninferiority of intravitreal ranibizumab compared to PRP for treatment of PDR, and the data suggest some potential benefits of ranibizumab over laser in terms of less effect on peripheral vision, better central macular thickness, and lower rates of vitrectomy, as mentioned above. These findings may lead to a paradigm shift in the treatment of PDR, although the frequency of injections and long-term follow-up intervals have yet to be established. PRP laser will remain an important treatment modality for PDR due to concerns about the burden of injections and those patients unable to return for frequent follow-up.
At our institutions, we treat patients with DME and concomitant DR with intravitreal anti-VEGF injections. We don’t routinely treat patients with NPDR without macular edema with intravitreal anti-VEGF injections. For our patients with PDR, we have a frank discussion regarding the results of Protocol S, and discuss the options of intravitreal anti-VEGF or PRP or combining both, deciding with the patient which treatment to pursue. For the majority of patients with PDR, we recommend initial treatment with anti-VEGF injections and then consider adding PRP laser at a subsequent visit if the burden of visits and injections is too difficult for the patient. Ultimately, we provide the patient with an individualized treatment plan based on the available medical evidence, in order to prevent progression of their proliferative diabetic retinopathy.
Looking towards the future, the treatment intervals and follow-up required to maintain improvements in diabetic retinopathy and proliferative DR will need to be determined, but long-acting anti-VEGF agents and delivery methods have the potential to transform the diabetic retinopathy treatment landscape. REVIEW
Dr. Greven is an assistant professor of ophthalmology at Wake Forest University School of Medicine Eye Center in Winston-Salem, and Dr. Do is a professor of ophthalmology at The Byers Eye Institute, Horngren Family Vitreoretinal Center, Department of Ophthalmology, Stanford University School of Medicine in Palo Alto.
Dr. Greven may be reached at margaret.greven@gmail.com. Dr. Do may be contacted at dianado@stanford.edu.
1. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789-801.
2. Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy: Long-term outcomes of the phase III RIDE and RISE Trials. Ophthalmology 2015;122:2504-2413.
3. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:6:1064–1077.
4. Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol 2013;131:1033-1040.
5. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. JAMA 2015;314:20:2137. doi:10.1001/jama.2015.15217.
6. Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122:10:2044.
7. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. Ophthalmology 2016;123:6:1351-1359.
8. Bressler SB, Liu D, Glassman AR, et al. Change in diabetic retinopathy through 2 years: Secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmology 2017;135:6:558-568.
9. Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology 2010;117:1078-1086.
10. Ip MS, Zhang J, Ehrlich JS. The clinical importance of changes in diabetic retinopathy severity score. Ophthalmology 2017;124:5:596-603.



