In February, the U.S. Food and Drug Administration approved the Tecnis Eyhance and Eyhance Toric II intraocular lenses (Johnson & Johnson Vision) for implantation in cataract patients in the United States. The new refractive surface design slightly increases the depth of focus compared to a standard monofocal. The company says this improves intermediate vision, while distance vision remains similar to that achieved with a standard monofocal. J&J Vision also states that the Eyhance lenses deliver 30 percent better image contrast in low light at 5 mm than a typical monofocal lens. At the same time, the company reports a low incidence of dysphotopsias, comparable to that associated with the previous Tecnis one-piece monofocal. Both Eyhance lenses are available in 0.5-D increments ranging from +5 D to +34 D.
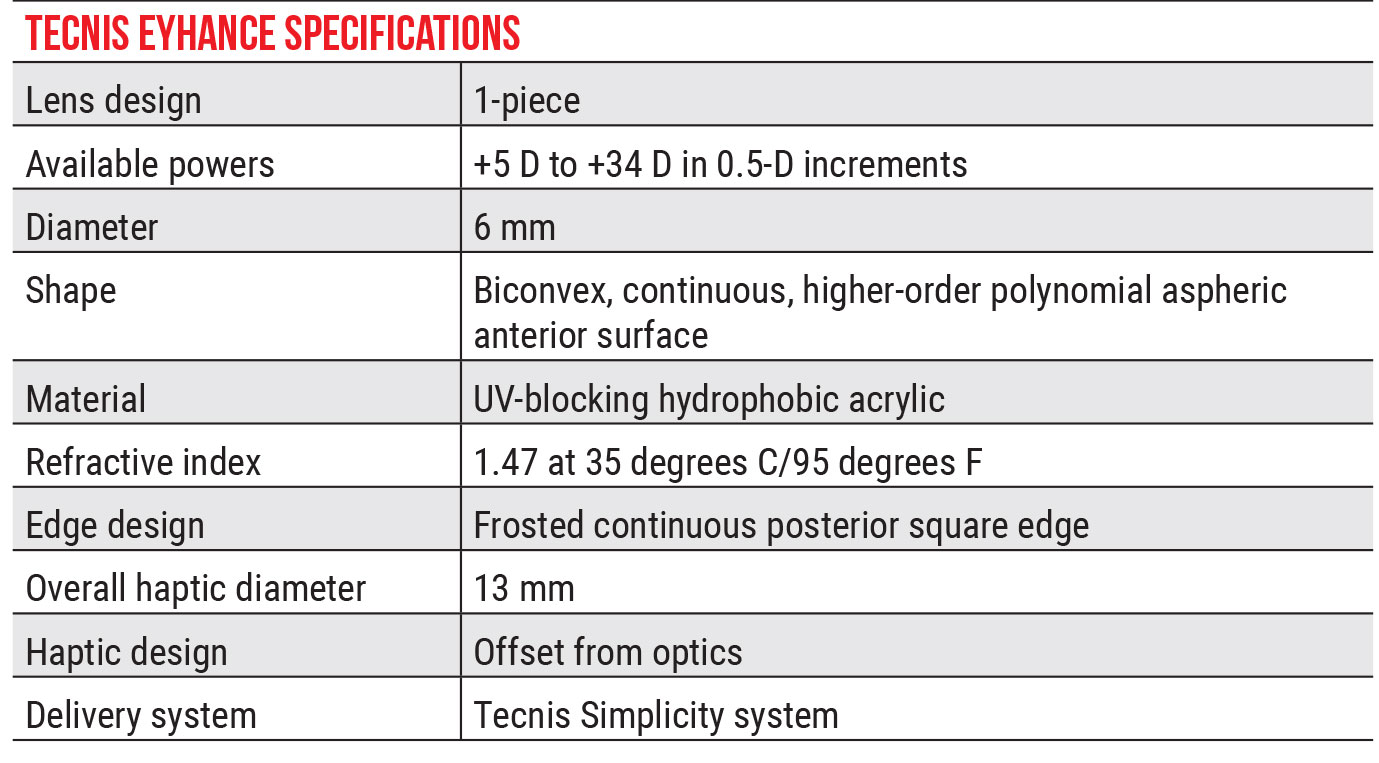 |
| Click table to enlarge. |
At least five published studies have compared the vision gained from an Eyhance lens to the previous Tecnis monofocal.1-5 All five studies found better intermediate vision with the Eyhance lens. Two studies also noted greater spectacle independence with the Eyhance;1,2 one noted a better tolerance for residual refractive error;1 and at least one study found better near vision, as well.4 The most frequently reported adverse event that occurred during the company’s SENSAR clinical trial was cystoid macular edema, which occurred at a rate of 3.3 percent.
Douglas D. Koch, MD, a professor and Allen, Mosbacher and Law Chair in Ophthalmology at Baylor College of Medicine in Houston, consults for Johnson & Johnson Vision; he says he’s watched and consulted on the evolution of this lens for a number of years. “It’s a very interesting design that consists of a continuous, higher-order aspheric surface,” he explains. “The result of this design is that there is a very slight, gradual central steepening. The laboratory data the company produced shows that this new design provides about one line of additional intermediate and near vision, with the modulation transfer function [a measurement of the optical performance potential of a lens] at distance being very close to that of the standard ZCB00 lens, particularly with larger pupil sizes.”
Professor Peter Szurman, chief physician at the Sulzbach Eye Clinic of the Knappschaft Hospital Saar in Sulzbach, Germany, has implanted many of the Eyhance lenses since their approval in Europe about two years ago. (He has no financial ties to the lens or to Johnson & Johnson Vision.) “Until now, cataract patients have had to choose between a presbyopia-correcting multifocal IOL—with all its advantages and disadvantages—and a standard monofocal IOL with a limited focal range,” he notes. “The Tecnis Eyhance is a breakthrough technology because, for the first time, a high-quality monofocal IOL offers extended depth of focus. To me, the Eyhance is a high-quality option for ordinary cataract management.
“Intermediate visual acuity is difficult to measure in daily eye-care practice,” he continues. “However, from the patient’s perspective, intermediate visual acuity is very important for daily life activities, such as seeing sharply when looking at a smartphone or the dashboard while driving. There are numerous everyday activities within an arm’s length. The Tecnis Eyhance increases freedom from spectacles at this important intermediate distance and thus the quality of daily life of my patients.”
Dr. Koch notes that the Eyhance lenses aren’t classified as extended depth-of-focus lenses in the United States. “The FDA has specific criteria for what constitutes an EDOF lens, and this lens hasn’t undergone the FDA-monitored testing needed to get this classification,” he explains. “However, the lens design does provide more intermediate vision and slightly more near vision than a standard monofocal. That’s an advantage, because it will be billed as a standard monofocal lens. A patient coming in for routine cataract surgery who doesn’t want—or can’t afford—an EDOF lens can get this lens and get a little bit more near vision. That’s a nice plus.
“I think the toric version will be a huge hit,” he adds. “Doctors can upcharge because it’s a toric, and the patient will get a little more intermediate and near vision. These lenses will also be good for patients with ocular pathology for whom you might be uncomfortable about implanting a lens that splits the light. Meanwhile, if the patient really wants even more intermediate and near vision, we still have the option of choosing an EDOF lens or multifocal or trifocal.”
In addition to the new refractive design, the lenses feature a new squared and frosted haptic intended to stabilize the lens and prevent toric lens rotation. Dr. Koch notes that the squared and frosted haptics are also used in the Tecnis Toric II (ZCU) model. “That’s a very stable lens,” he says. “I’ve been using it for well over a year and I haven’t had a single rotation with it. I’m sure they’ll be using that haptic design in all the lenses they’re bringing to market going forward. It’s rock solid.”
Professor Szurman says that he has tended to avoid implanting toric lenses in the past because of the insufficient rotational stability of some of them. “The frosted surface texture and more squared haptic design of the Eyhance lenses are the way to go to increase rotational stability and allow more patients to benefit from toric IOLs,” he says.
Both lenses are delivered into the eye using the new Tecnis Simplicity delivery system, designed to make implantation as easy and contamination-free as possible. “The preloaded delivery system allows my assistant nurse to easily and safely prepare the IOL within seconds, with no loading errors,” says Professor Szurman. “I get a ready-to-use system that shortens my OR time and allows for a smooth and controlled implantation.”
Dr. Koch says the Simplicity delivery system will also be used for the ZCB00 Tecnis lens. “As the name suggests, it’s simple to use,” he says. “You just inject a little BSS through a small portal and screw the lens in. It glides into the eye very smoothly. It’s about as foolproof as it can be.”
Asked whether he thinks this new monofocal design might result in the Eyhance intraocular lens replacing standard monofocal lenses, Dr. Koch says it’s possible. “It’s essentially like getting something for nothing,” he says. “There’s a trivial change in distance vision if the patient has a small pupil, but it’s not perceptible, per my colleagues in Europe. To provide patients with one additional line of acuity without undercutting distance vision will be a nice plus. To me, it’s a can’t-lose proposition from the patient’s standpoint.”
“It’s important to clearly communicate to our patients that the Tecnis Eyhance is not a presbyopia-correcting refractive IOL, but an enhanced monofocal IOL,” adds Professor Szurman. “In my opinion, it’s a good option for all patients who are risk-averse or unsuitable for refractive cataract surgery, but who still want to maximize their functional vision. I routinely offer the Tecnis Eyhance to all patients undergoing monofocal IOL implantation. However, I exclude patients with vision-limiting ocular conditions other than cataract.”
The Eyhance lens has been available in Europe since February 2019 and became available in Latin America and Canada in the summer of 2020. The Eyhance Toric II will be launching in Europe and Canada later this year.
1. Unsal U, Sabur H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int Ophthalmol. 2021;41:1:273-282.
2. Mencucci R, Cennamo M, Venturi D, et al. Visual outcome, optical quality, and patient satisfaction with a new monofocal IOL, enhanced for intermediate vision: Preliminary results. J Cataract Refract Surg. 2020;46:3:378-387.
3. Eguileor B, Martinez-Indart L, Alday N, et al. Differences in intermediate vision: Monofocal intraocular lenses vs. monofocal extended depth of focus intraocular lenses. Arch Soc Esp Oftalmol. 2020;95:11:523-527.
4. Yangzes S, Kamble N, Grewal S, et al. Comparison of an aspheric monofocal intraocular lens with the new generation monofocal lens using defocus curve. Indian J Ophthalmol. 2020;68:12:3025-3029.
5. Auffarth GU, Gerl M, Tsai L, et al. Clinical evaluation of a new monofocal intraocular lens with enhanced intermediate function in cataract patients. J Cataract Refract Surg 2021;47:2:184-91.
In Brief Trefoil Begins Second Phase II STORM Trial
|
Bright Light May Help Glaucoma Patients Sleep
Glaucoma patients who are exposed to bright light during the day may have improved sleep and an increased melanopsin-dependent pupil response after just four weeks, a pilot study in Frontiers in Neurology reports.1
The multinational research team composed of researchers from Switzerland, the United Kingdom and New Zealand suggests the change in pupil response might indicate that melanopsin activity in viable intrinsically photosensitive retinal ganglion cells can adapt to different light levels, if sustained over a period of time.
Their study enrolled 20 glaucoma patients without severe vision loss and considered how 30 minutes of daily bright light exposure from a table-based light box placed in their homes affected pupil constriction, circadian rest-activity cycles, sleep, relaxation, alertness and mood.
Participants continuously wore an activity monitor and self-assessed sleep quality, well-being and visual comfort for several days before and during the four weeks of daily 10,000 lux polychromatic bright white light exposure.
Individuals underwent pupillometry at baseline and on the last day of the study.
Following light exposure, participants showed a much greater post-illumination pupil response and had better quality of sleep. Additionally, researchers report that they found no significant changes in the participants’ 24-hour rhythms or sleep parameters.
The study demonstrated that even a relatively short duration of added light exposure in a room is beneficial and also supports the general advice that the elderly should go outside for half an hour each morning, the researchers suggest.
While glaucoma patients can never recover the vision lost from damaged retinal ganglion cells, they may be able to maintain a robust day–night cycle and concomitant good circadian entrainment which helps maintain high sleep quality, the investigators explain.
1. Kawasaki A, Udry M, Wardani ME, Münch M. Can extra daytime light exposure improve well-being and sleep? A pilot study of patients with glaucoma. Front Neurol 2020;11:584479.
Correction |



