Over the past five years, our specialty has witnessed several significant changes in the technologies available to us in the operating room. Though these innovations come from different parts of the technology realm, they each aim to improve surgeons’ performance and outcomes. Such innovations include new models of surgical microscopes and smaller, more efficient vitrectomy devices. Looking ahead, robotics will be present in the operating room sooner than we expect and may allow the surgeon to perform delicate tasks without injuring adjacent tissues. Here, we’ll discuss some of the latest inventions.
Visualization
One area that’s made strides in recent years is our ability to visualize retinal procedures. This new point-of-view includes 3-D systems like that from Alcon/TrueVision and real-time, intraoperative optical coherence tomography.
The Ngenuity 3-D system uses a high-dynamic-range camera and a high-resolution 4K monitor. It provides the surgeon with very good image depth and magnification power, while maintaining a wide field of view and comfortable ergonomics for long surgeries.1 The digital image system and regulation in the aperture of the camera can display a usable image while using lower light intensity, which could mean less retinal phototoxicity for patients. The manipulation of the digital image with the use of different image filters to improve the color and contrast at specific moments during the surgery is another possibility that will be explored in the near future.
One significant benefit of a 3-D viewing system is that it allows the surgeon to teach residents and fellows surgical techniques, since it allows the operating team to see what the surgeon sees (Figure 1). Also, an assistant can help by designating targets and defining paths using a computer mouse.
Many retinal surgeons around the world seem interested in the technology; the latest Preferences and Trends Survey of the American Society of Retinal Specialists in 2018 reported that 15 to 20 percent of surgeons have used the 3-D system and found it useful. Furthermore, 35 to 50 percent said that they plan to purchase the equipment in the future.
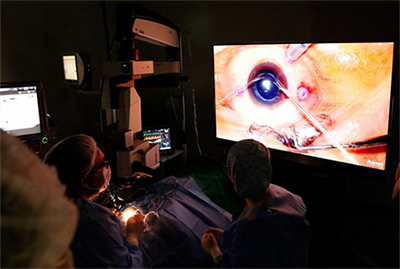 |
| Figure 1. Heads-up surgery may help improve documentation as well as assist in the training of other surgeons. |
Also in the imaging arena, intraoperative OCT has made strides.6 Systems such as the iOCT (Haag-Streit), EnFocus (Leica) and the Rescan 700 (Carl Zeiss Meditec) can make internal limiting membrane peeling, management of adhesions and visualization of a tractional detachment easier, as well as give a better view of the subretinal space (Figures 2 and 3).
The advent of swept-source OCT, with its longer wavelength compared to SD-OCT, improved the study of pre- and postoperative retinal status.7,8 This characteristic of SS-OCT allows it to visualize through some media opacities and to reduce RPE laser scattering. After macular hole surgery, SS-OCT appears to be a novel way to assess retinal status in a gas-filled vitreous cavity, and provides a high-quality image. Therefore, with an early assessment of macular hole closure, patients may need to be in a face-down position for less time.7 Certainly, image quality and acquisition latency from devices such as the intraoperative OCT have much to improve. However, there wouldn’t be any innovation without any initial effort.
Instruments
Alongside a new view of the procedure are new ways to manipulate the posterior segment’s structures.
• 27-ga. vitrectomy cutters. The 27-ga. vitrectomy system was introduced in 2010, but, to date, has not been adopted by most retinal surgeons.2 This is shown by the 2017 ASRS PAT Survey in which about 70 percent of surgeons reported not using it, and 20 percent say they use it for only 5 percent of their cases.
Despite creating self-sealing incisions and the promise of lower frequency of hypotonia, vitreous prolapse and endophthalmitis, disadvantages such as reduced luminance from a smaller-gauge light probe and worse vitreous aspiration rates may have delayed the widespread adoption of this technology.
With technological improvements, different light sources such as xenon, mercury vapor or LEDs were introduced. These light sources decreased the risk of phototoxicity while still yielding good illumination. This efficient lighting allows better visualization in 27-ga. surgeries, making the option more attractive to surgeons. What’s more, a 3-D surgical viewing system can optimize the illumination of the lower-gauge cutting devices, thanks to the aperture control of the camera and its ability to use less light. Another critical step toward improved efficiency with the 27-ga. system is the use of high-cut-rate technology. With a higher cutting rate, the aspirated vitreous has a lower viscosity, allowing a better rate of aspiration.
Another recent innovation is two-dimensional cutting. TDC is a technology developed by Dutch Ophthalmic Research Center that features two cutter openings inside the guillotine shaft. This design allows a vitreous cutting action in both the forward and backward movements. The main benefits of this technology include cutting rates of up to 16,000 cpm, increased flow rate and reduced retinal traction. Other companies also use techniques to increase duty cycle and improve system efficiencies, such as the Bi-Blade Dual Port (Bausch + Lomb) and the Hypervit Dual-Blade (Alcon) that can generate up to 20,000 cpm.
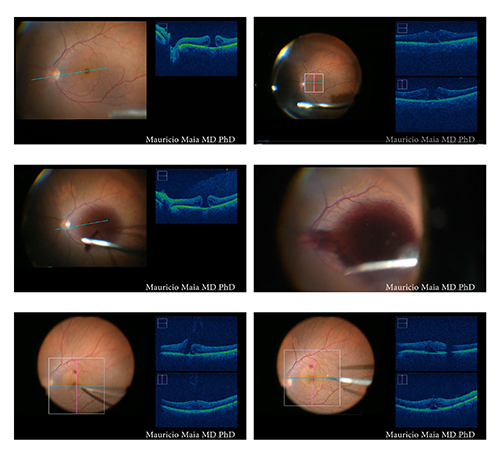 |
| Figure 2. Intraoperative optical coherence tomography during macular hole surgery imaged during an açaí dye protocol. Upper left: One line raster. Upper right: Macular cube. Middle left and middle right: Injection of açaí dye. Bottom left: OCT during peeling. Bottom right: OCT during fluid-air exchange, with intraoperative verification of macular hole closure. |
Due to the improvements in the features and safety of the 27-ga. system, the indications for its use have expanded. Today, it can be used in nearly all cases of retinal surgery and it’s especially useful in cases of diabetic retinopathy with multiple areas of fibrovascular proliferation and retinal traction. Thanks to its size, the 27-ga. probe can easily enter small spaces for membrane dissection, reducing the need for bimanual techniques.3 Despite the companies’ and surgeons’ best efforts, though, the 27-ga system still has limitations, with reduced stiffness of the instruments and fragile tools for longer complex surgeries.
Some patients who are receiving multifocal IOLs, and who have symptomatic floaters, find their floaters to be more bothersome, and some surgeons feel that vitrectomy is a possible option for these patients. For those cases of floaters in patients with excellent visual acuity, a 27-ga vitrectomy could be a suitable procedure due to the smaller scleral wound and the possibility for reduced leakage. This sutureless procedure may decrease the risk for refractive errors postop and also may reduce the risk of endophthalmitis (Figure 4), though more data on this is required.4,5
• New models of instruments and vitrectomy cutters. The latest technology in the field of vitrectomy cutters is the hypersonic probe developed for the Stellaris Elite (Bausch + Lomb), which is available for the company’s 23-ga. system. During vitrectomy, this probe uses hypersonic vibrations that reach about 1.7 million per minute to liquify adjacent vitreous. With this technology, the aspiration port is open at all times, allowing constant aspiration flow and, consequently, greater stability in vitreous removal, even in severe cases such as pediatric vitrectomies.
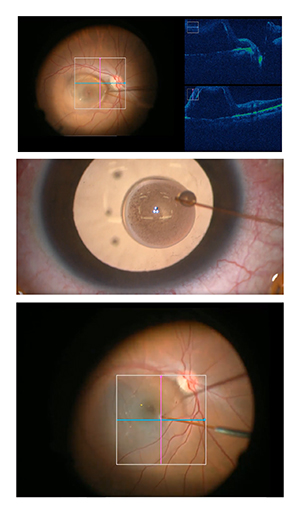 |
| Figure 3. Intraoperative optical coherence tomography to assist with surgery in the subretinal space in UNIFESP Stem Cell protocol, Sao Paulo, Brazil. Top: iOCT during the creation of a subretinal space. Middle: 40-ga. needle with stem cells. Bottom: Injection of stem cells into subretinal space. |
Technique Assistance
In addition to visualization, implementation of technologies in different areas—such as robotics and pharmacology—has enhanced our surgical techniques and could improve surgical outcomes.
Experimental models of robotic instruments have demonstrated that it may be possible to reduce surgeon hand tremor in order to access specific retinal depths precisely—including intravascular spaces. Robotics may also be able to prevent inadvertent retinal touch.9 In addition, a procedure performed by a surgeon-controlled robot—already a reality in surgical specialties such as gastrointestinal surgery and urology—is in a human trial, and has already performed 12 surgeries.10
Currently, one of the possible limitations of this technology lies in the massive number of variables and patterns during surgery that a human can identify and react to faster than the algorithm. There’s also the constant fear of software failure during the operation.
In the realm of pharmacology, research into novel anti-vascular endothelial growth factor agents that could be used before surgery to reduce bleeding; new dyes, such as an açaí-fruit extract (Figure 2),11,12 that could be used during the operation to improve the identification of ocular structures; and the development of sustained-release drug implants all show potential to enhance both our techniques as well as visual outcomes. Research is also underway on a new vitreous substitute that may decrease the need for face-down positioning after certain retinal procedures.
Visual and Retinal Recovery
Researchers are also investigating ways to assist retinal and/or visual recovery and regeneration. Due to the level of difficulty of these projects, however, their results and numbers are still not statistically robust. However, they’re still very important, since they’re encouraging more studies in their areas. Embryological, genetic and technological projects stand out in the search for retinal and visual recovery.
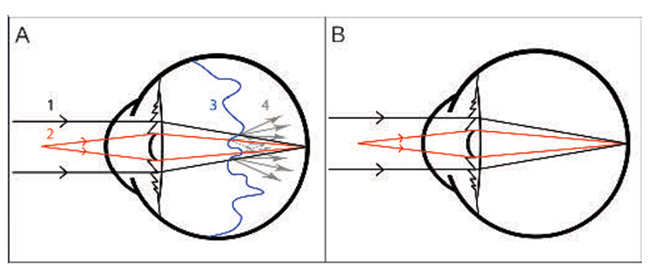 |
| Figure 4. The benefits of small-gauge vitrectomy for floaters are minimal wound leakage, less postop inflammation and complications. (A) Drawing showing an eye with a multifocal IOL and posterior vitreous detachment. The light rays first pass through the cornea and then the IOL. The high density of the PVD causes dispersion of the light rays. (1) The light rays in black represent distance vision. (2) The light rays in red represent near vision. (3) The wavy blue line represents vitreous detachment. (4) The gray arrows show dispersion of the light rays when they pass through the dense vitreous, causing halos floaters and blurred vision. (B) The drawing shows the light rays reaching the retina without interference from the vitreous detachment after PPV, indicating the potential for good near and distance vision without glasses. (Navarro RM, Maia A, Arevalo F, et al. J Ophthalmol. 2015; 2015:156910.) |
Embryology has been an exciting tool in retinal recovery, mainly with the use of stem cells (recently covered in the June 2018 Retinal Insider by Peter Bracha, MD, and Thomas A. Ciulla, MD). Currently, however, amniotic transplantation has been questioned as a tool for recurrent macular holes (NCT03528122). The treatment using embryological therapy suffered from lack of safety data until recently, and it’s important to emphasize that such studies should be ethical and conscientiously analyzed, and their results carefully published, to avoid a frenzy from the media and desperate patients.
Providing stronger evidence, at least in specific applications, are genetic therapies, exemplified by Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics). Luxturna’s successful trial and subsequent approval caught the world’s attention and encouraged further studies. The main obstacle, however, to a greater emphasis on gene therapy is the high cost of the treatment. With a larger number of clinical trials, and a more significant number of companies supporting the research, the production cost could possibly be lowered, and the feasibility of this type of treatment could increase.
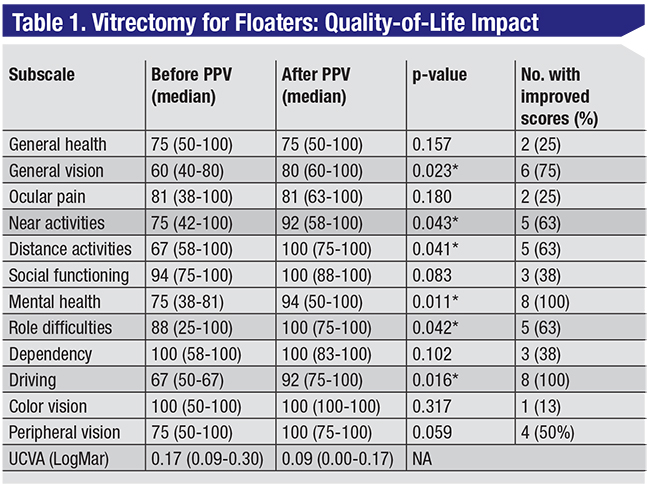 |
| Visual function questionnaire score results before and after vitrectomy. * indicates statistically significant result. Table data adapted from: Navarro RM, Maia A, Arevalo F, et al. J Ophthalmol. 2015:156910. |
Another trend in visual rehabilitation is the use of retinal prostheses (reviewed in the June 2017 Retinal Insider by Derrick L. Cheng, MD, David A. Borton, MD, and Paul B. Greenberg, MD). The current improved access and ability to manipulate different retinal sites (epiretinal, subretinal, suprachoroidal), as well as improved computer processing, make the implantation of these prostheses a promising area of research. With all of these strategies for vision restoration or recovery, it’s essential to reiterate the need to conduct and publish this research ethically. We must remember that these studies usually involve individuals with severe vision loss, and their desperate need to believe in a “miracle” could make them jump at the nearest study or therapy.
Trends in vitreoretinal surgery go hand-in-hand with scientific evidence. The interplay between technology and techniques can start a trend or reveal a new solution to an old problem. For instance, new visualization systems could allow us to document surgery differently, and therefore improve our ability to critically analyze each new technological trend as it works its way through the pipeline. In this way, using scientific evidence, surgeons will be able to make the best decisions regarding which new technology to use for their surgeries. REVIEW
Dr Maia is a professor of ophthalmology at Federal University of São Paulo-UNIFESP. He can be reached at mmaia@unifesp.br. Dr. Urias and Dr. Pereira are vitreoretinal fellows and postgraduate students at UNIFESP. Dr. Brant and Dr. Caiado are also postgraduate students at UNIFESP. Dr. Belfort Jr. is the chair of the ophthalmology department of UNIFESP.
Dr. Maia is a consultant for Alcon, Allergan, Bausch + Lomb, Bayer, Kemin and Novartis. Dr. Belfort is a consultant to Abbvie, Alcon and Allergan, and has received grants from Novartis, Ophthalmos and Roche.
1. Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures : An experimental and clinical study. Retina 2016;36:1:137-47.
2. Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 2010;117:1:93-102.
3. Cruz-Iñigo YJ, Berrocal MH. Twenty-seven-gauge vitrectomy for combined tractional and rhegmatogenous retinal detachment involving the macula associated with proliferative diabetic retinopathy. Int J Retin Vitr 2017;9;3:38.
4. Lin Z, Moonasar N, Wu RH, Seemongal-Dass RR. 27-gauge vitrectomy for symptomatic vitreous floaters with topical anesthesia. Case Rep Ophthalmol 2017;20;8:1:35-39.
5. Lin Z, Zhang R, Liang QH, et al. Surgical outcomes of 27-gauge pars plana vitrectomy for symptomatic vitreous floaters. J Ophthalmol 2017;2017:549.
6. Carrasco-Zevallos OM, Keller B, Viehland C, et al. Live volumetric (4D) visualization and guidance of in vivo human ophthalmic surgery with intraoperative optical coherence tomography. Sci Rep 2016;19;6:31689 [online journal].
7. Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express 2012;3:11:2733-51.
8. Kikushima W, Imai A, Toriyama Y, Hirano T, Murata T, Ishibashi T. Dynamics of macular hole closure in gas-filled eyes within 24 hours of surgery observed with swept source optical coherence tomography. Ophthalmic Res. 2015. doi:10.1159/000368437
9. Roizenblatt M, Edwards T, Gehlbach PL. Robot-assisted vitreoretinal surgery: current perspectives. Robot Surg Res Rev 2018;5:1-11.
10. Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nature Biomedical Engineering. 2018;18:2:649-56.
11. Chen J, Ferreira MA, Farah M, et al. Posterior hyaloid detachment and internal limiting membrane peeling assisted by anthocyanins from açaí fruit (Euterpe oleracea) and 10 other natural vital dyes: Experimental study in cadaveric eyes. Retina 2013;33:1:89-96.
12. Badaro E, Novais EA, Penha FM, Maia M, Farah ME, Rodrigues EB. Vital dyes in ophthalmology: A chemical perspective. Curr Eye Res 2014;39:7:649-58.



