Do you know the latest information on medications with ocular side effects recently approved by Food and Drug Administration for use in children? These drugs can cause everything from blurred vision and photophobia to cataracts, so it pays to be aware of their safety profiles. Here, we’ll provide insights on these therapeutics and the associated ocular complications to be on the lookout for in your pediatric patient population.
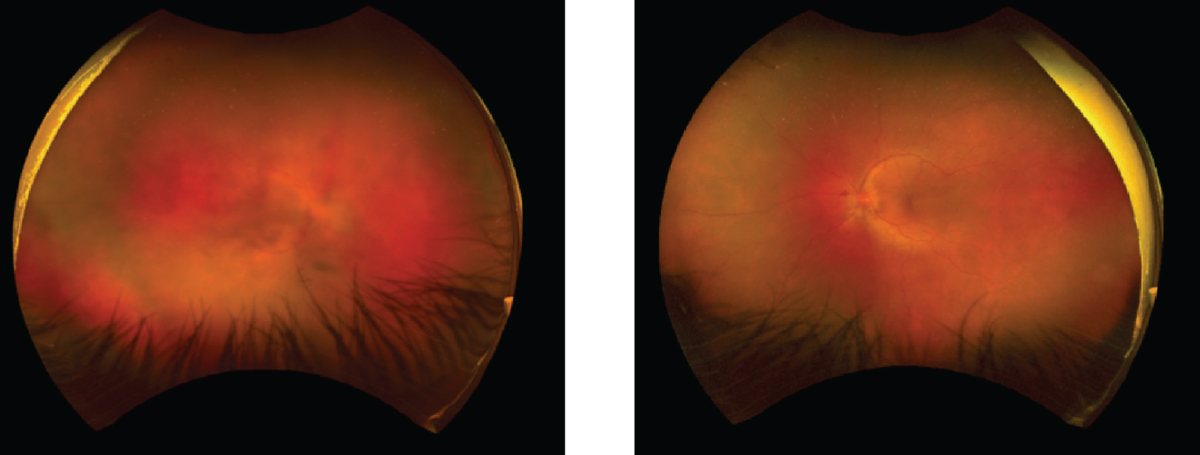 |
| Figure 1. Wide-field photographs (Optos) showing panuveitis seen in a 40-year-old patient with cancer drug-induced uveitis (nivolumab). Fluorescence angiography showed late peripheral and macular leakage. Photo: Jila Noori, MD. |
The Medications
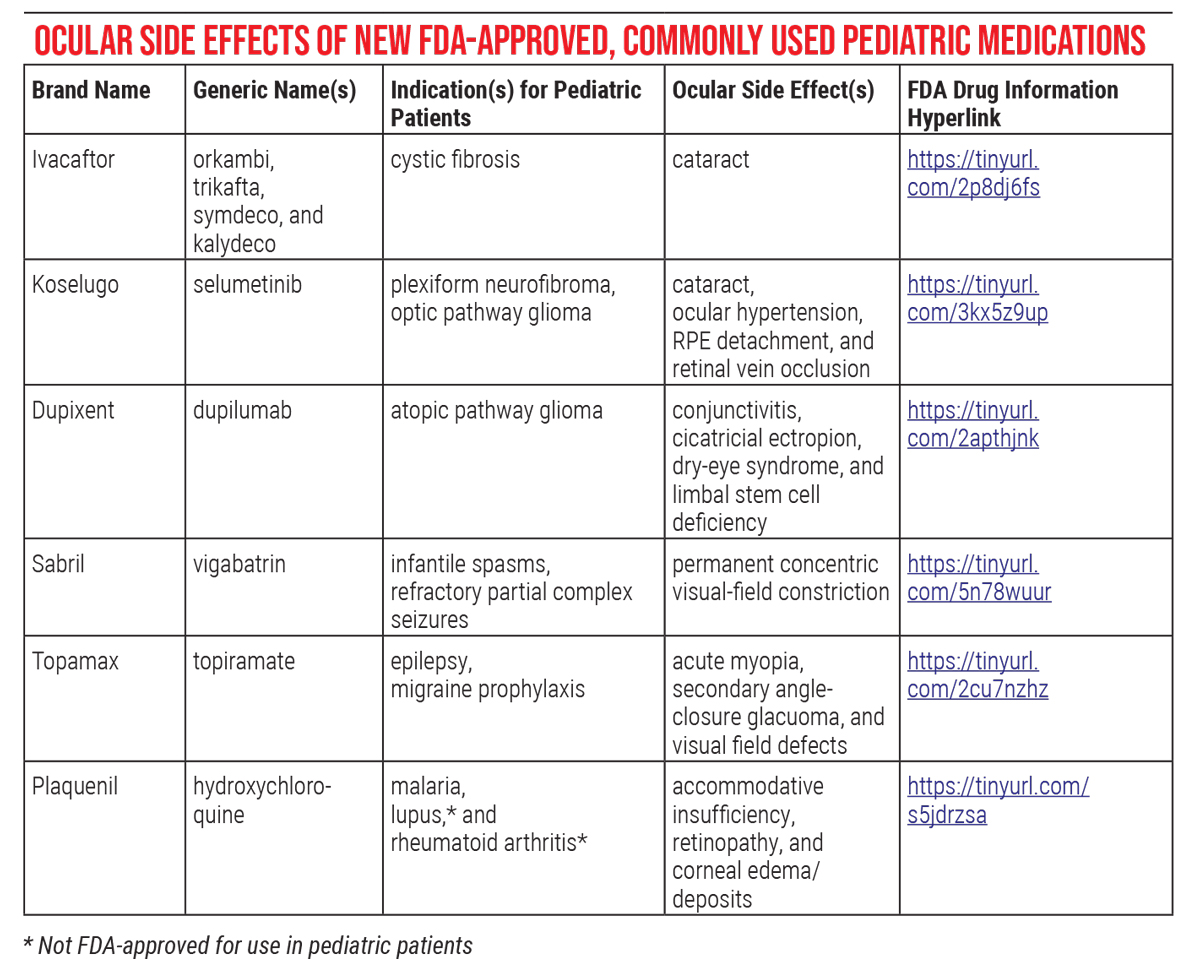
The table on the following page summarizes the recently approved medications and a few other commonly used pediatric medications with ocular toxicities. Following are the medications and their toxicity issues to be aware of.
• Cystic fibrosis medications: Elexacaftor/Ivacaftor/Lumacaftor/Tezacaftor (Trikafta, Kalydeco, Symdeco, and Orkambi). Elexacaftor, ivacaftor (Kalydeco), lumacaftor, and tezacaftor are cystic fibrosis transmembrane conductance regulator potentiators. CFTR potentiators improve chloride ion transport in patients with cystic fibrosis caused by specific gene mutations. Elexacaftor/ivacaftor/tezacaftor (Trikafta), ivacaftor/tezacaftor (Symdeco), and ivacaftor/lumacaftor (Orkambi) are combination drugs in which multiple CFTR potentiators are formulated into a single agent to increase efficacy. The FDA approved ivacaftor for patients with CF 4 months of age and older with at least one responsive mutation in the cystic fibrosis gene. The FDA has also approved combination CFTR potentiator drugs, including ivacaftor/lumacaft for children 2 years of age and older and elexacaftor/ivacaftor/tezacaftor and ivacaftor/tezacaftor for children 6 years of age and older.
—Ocular side effects. Investigators have reported non-congenital cataracts (cortical and subcapsular) in pediatric patients treated with ivacaftor mono- and combined therapy.1 The product information says that “although other risk factors [for cataract development] were present in cases (such as corticosteroid use and radiation exposure), a risk attributable to treatment with ivacaftor cannot be excluded.”
Due to this risk, the manufacturer recommends that ophthalmologists perform baseline and follow-up examinations in pediatric patients on ivacaftor (Symdeco [package insert], Boston, Vertex Pharmaceuticals). For babies born to breastfeeding mothers taking ivacaftor, the FDA also recommends examination for cataracts.2 Investigators have not yet figured out how ivacaftor induces cataracts, and no published protocol supplies a suggested frequency for follow-up exams. However, pediatric ophthalmologists might consider screening younger children more frequently, given the critical period of visual development and its potential associated risk of deprivation amblyopia.
 |
| Click image to enlarge. |
— Management of complications. The main management strategies for these drugs’ unwanted ocular effects are refractive correction, amblyopia management and cataract surgery. Ophthalmologic evaluation for children on cystic fibrosis medications should include age-appropriate visual acuity testing, biomicroscopy (standard or portable slit lamp), and full retinal examination. Cataracts that are less than 3 mm in diameter or of partial density may be observed. In older children, ophthalmologists should consider cataract surgery for any opacity causing a decrease in quality of life. After cataract surgery, ophthalmologists should initiate optical rehabilitation and amblyopia management.
• Selumetinib (Koselugo) for neurofibromas and optic pathway gliomas. Selumetinib is a drug that blocks the mitogen activator protein kinase (MAPK) pathway. Specifically, it inhibits mitogen-activated protein kinase enzymes (MEK), causing cell death and stopping tumor growth. Oncologists have used MEK inhibitors to treat metastatic melanoma and other cancers in adults. In 2020, selumetinib received FDA approval for the treatment of symptomatic, inoperable plexiform neurofibromas in patients with neurofibromatosis (NF) type 1 who are 2 years and older.3 Selumetinib also shows promise in treating pediatric patients with non-NF type 1 associated optic pathway gliomas,4 though it’s not approved by the FDA for this indication.
— Ocular complications. Patients treated with selumetinib have reported visual changes, including blurred vision and photophobia, and have developed cataracts and ocular hypertension.5 In adult patients treated with MEK inhibitors, the more severe ocular complications of retinal pigment epithelial detachment and retinal vein occlusion have been noted.6 Pediatric patients have also shown outer retinal separation.7 The RPE detachment associated with selumetinib is typically bilateral and symmetric. The patient’s symptoms can vary from nothing at all to blurred vision, altered color perception, shadows, light sensitivity, metamorphopsia and glare. Diagnosis of RPE detachment may require macular OCT. Separation occurs because of RPE degeneration secondary to MEK pathway inhibition. Under normal conditions, activation of the MEK pathway supports the RPE. The RPE detachment associated with selumetinib typically doesn’t result in irreversible loss of vision or eye damage and resolves with discontinuation of the medication.
Uveitis is another severe but rare ocular complication associated with MEK/BRAF inhibitor and other cancer drug treatments such as immune checkpoint inhibitors in adult patients.8 MEK/BRAF inhibitor-related uveitis causes severe uveal tract inflammation that may lead to irreversible vision loss. Figure 1 shows an example of cancer-drug-induced uveitis. The fundus photographs are from a 40-year-old adult patient with cancer-drug-induced uveitis due to treatment with nivolumab, an immune checkpoint inhibitor.9 Because uveitis is such a rare occurrence, it remains to be determined if there’s an association between uveitis and MEK inhibitors in the pediatric population. However, pediatric ophthalmologists should consider using extra vigilance in looking for signs or symptoms of uveitis in their patients being treated with selumetinib.
— Monitoring. The prescribing information recommends baseline ophthalmic assessments in pediatric patients starting selumetinib. The package insert also suggests ophthalmic exams at regular intervals during treatment and for any new or worsening visual changes. In patients with visual changes, ophthalmologic evaluation should include a best corrected visual acuity, intraocular pressure, and slit lamp fundoscopy. Physicians should also consider a macular OCT.
Ophthalmologists need more information to determine how often these ocular side effects occur in the pediatric population. There are no published screening protocols. For younger patients, detecting RPE detachment without macular OCT may prove challenging for pediatric ophthalmologists.
Currently, the main management options are cataract surgery and withholding or discontinuing the medication. The drug’s package insert suggests permanently discontinuing selumetinib for retinal vein occlusion and, in cases of RPE detachment, withholding selumetinib while checking optical coherence tomography assessments every three weeks until resolution and then resuming at a reduced dose. As uveitis is a rare complication, currently there are no guidelines for its management in these patients. Collaborative care nuanced to the disease specific to the patient is advised.
• Dupilumab (Dupixent). Dupilumab is an interleukin (IL)-4 receptor inhibitor administered by subcutaneous injection. It was recently FDA-approved for “the treatment of moderate to severe atopic dermatitis in pediatric patients aged six months to 5 years whose disease is not adequately controlled with topical therapies or when those therapies are not advisable.” Dupixent has already been approved for the treatment of the following indications:
- moderate to severe atopic dermatitis in patients six years of age and older;
- maintenance treatment of severe asthma in patients 12 years of age and older; and
- the treatment of eosinophilic esophagitis in patients 12 years of age and older weighing at least 40 kg.
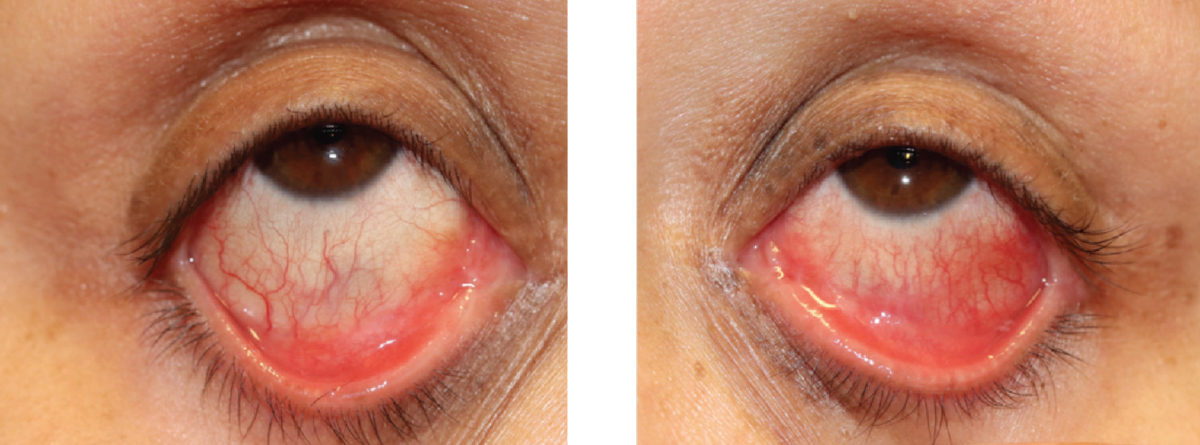 |
| Figure 2. External photos showing conjunctivitis and early symblepharon due to dupilumab use in a 28-year-old patient. Photo: Jeremy Tan, MD. |
— Ocular complications. A commonly reported ocular side effect noted in children and adults is conjunctivitis. In the adult literature, there have also been cases of symblepharon and cicatricial ectropion, dry-eye syndrome, and limbal stem cell deficiency.10,11 Researchers hypothesize that dupilumab-associated conjunctivitis might be due to goblet cell loss, heightened OX40 ligand activity, eosinophilia and increased Demodex infestation due to changes in the ocular surface environment.12-14 In the case of the limbal stem cell deficiency, the report speculated that it occurred because of continued use of the medication well after the appearance of symptoms.
Figure 2 shows conjunctivitis and symblepharon developed in an adult patient treated with dupilumab. In this patient, both conjunctivitis and symblepharon improved but didn’t completely resolve with treatment.
In terms of monitoring recommendations, that’s still an open question. The need for and frequency of screening exams in the pediatric population require further investigation.
— Management of complications. Management consists of topical steroids and/or topical immunomodulators such as tacrolimus or cyclosporin, as well as discontinuing dupilimab in rare cases. Artificial tears and topical anti-histamines aren’t effective. Effective treatment stops the inflammatory process. It’s thought that topical steroids and/or topical immunomodulators prevent epithelial cell death by increasing goblet cells. — Management of complications. Management consists of topical steroids and/or topical immunomodulators such as tacrolimus or cyclosporin, as well as discontinuing dupilimab in rare cases. Artificial tears and topical anti-histamines aren’t effective. Effective treatment stops the inflammatory process. It’s thought that topical steroids and/or topical immunomodulators prevent epithelial cell death by increasing goblet cells.
In conclusion, new FDA-approved medications have significant benefits for pediatric patients with a variety of diseases. However, ophthalmologists must be aware of potential ocular complications, and develop proper screening and treatment protocols when necessary to preserve vision in our patients. In addition, it is vital to keep an open line of communication with the managing specialist(s) of patients with these disorders to optimize care.
Dr. Collinge is an assistant professor in the Department of Pediatrics of the University of Connecticut School of Medicine. She has no financial interest in any of the products discussed in the article.
Mr. Vaclaw is an MS4 at the Oklahoma College of Medicine. Dr. Yanovitch is a clinical professor of Pediatric Ophthalmology and Strabismus and director of Medical Student Education at the Dean McGee Eye Institute/University of Oklahoma College of Medicine-Department of Ophthalmology.
1. Talamo Guevara M, McColley SA. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Opin Drug Saf 2017;16:11:1305-1311.
2. Taylor-Cousar JL. CFTR modulators: Impact on fertility, pregnancy, and lactation in women with cystic fibrosis. J Clin Med 2020;9:2706.
3. Gross AM, Wolters PL, Dombi E, et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med 2020;382:15:1430-1442. Erratum in: N Engl J Med. 2020;383:13:1290.
4. Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, Campagne O, Banerjee A, Gururangan S, Kilburn LB, Goldman S, Qaddoumi I, Baxter P, Vezina G, Bregman C, Patay Z, Jones JY, Stewart CF, Fisher MJ, Doyle LA, Smith M, Dunkel IJ, Fouladi M. A phase II trial of selumetinib in children with recurrent optic pathway and hypothalamic low-grade glioma without NF1: A Pediatric Brain Tumor Consortium study. Neuro Oncol 2021;23:10:1777-1788.
5. Stjepanovic N, Velazquez-Martin JP, Bedard PL. Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol 2016;27:6:998-1005.
6. Klesse LJ, Jordan JT, Radtke HB, et al. The use of MEK inhibitors in neurofibromatosis type 1-associated tumors and management of toxicities. Oncologist 2020;25:7:e1109-e1116.
7. Avery RA, Trimboli-Heidler C, Kilburn LB. Separation of outer retinal layers secondary to selumetinib. J AAPOS 2016;20:3:268-71.
8. Thurau S, Engelke H, McCluskey P, et al. Uveitis in tumor patients treated with immunological checkpoint- and signal transduction pathway-inhibitors. Ocul Immunol Inflamm 2021;1-7. (Online ahead of print.)
9. Iris Deitch-Harel MD, Eyal Raskin MD, Zohar Habot-Wilner MD, Ronit Friling MD, Amer R, Michal Kramer. Uveitis induced by biological agents used in cancer therapy. Ocular Immunol Inflamm 2021;29:7-8:1370-1374.
10. Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019;181:3:459-473.
11. Mehta U, Farid M. Dupilumab induced limbal stem cell deficiency. Int Med Case Rep J 2021;14:275-278.
12. Popiela MZ, Barbara R, Turnbull AMJ, et al. Dupilumab-associated ocular surface disease: Presentation, management and long-term sequelae. Eye (Lond) 2021;35:12:3277.
13. Barnett BP, Afshari NA. Dupilumab-associated mucin deficiency (DAMD). Transl Vis Sci Technol 2020;9:3:29. Erratum in: Transl Vis Sci Technol 2020;9:8:21.
14. Maudinet A, Law-Koune S, Duretz C, et al. Ocular surface diseases induced by dupilumab in severe atopic dermatitis. Ophthalmol Ther 2019;8:3:485-490.




