Training
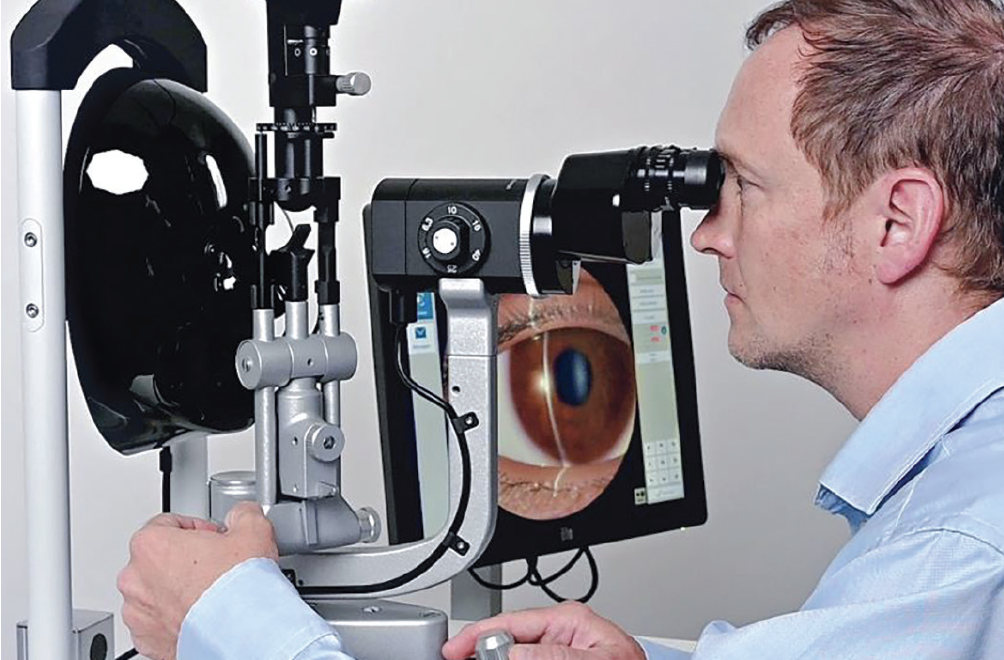 |
Slit Lamp Simulator Goes Gonio
Haag-Streit says its EyeSi Slit Lamp Simulator now offers a gonioscopy module.
The simulator integrates virtual reality technology into the original hardware of a BQ 900 model slit lamp and simulates all functions of the real slit lamp, the company says. Looking through the simulator’s biomicroscope and using the slit lamp controls, trainees see the visualization of a virtual patient’s eyes. Software modules provide various patients presenting with healthy eyes or clinically relevant pathologies. For visualization of the iridocorneal angle, a gonioscopy lens mimic is now available with the new gonioscopy module. Haag-Streit says pathologies included in the new module include pseudoexfoliation syndrome, iris nevus and iris melanoma. To practice grading the angle, the Shaffer-Kanski and Spaeth classification systems are available.
For information, visit https://www.vrmagic.com/medical-simulators/eyesi-slit-lamp.
Ocular Surface
Generic Restasis Approved
Viatris recently announced that its subsidiary, Mylan Pharmaceuticals, has received approval from the FDA for its Abbreviated New Drug Application (ANDA) for Cyclosporine Ophthalmic Emulsion 0.05%, the first generic version of Allergan’s dry-eye drug Restasis. The company says the drug will be “launched immediately.”
For information, visit viatris.com.
Eyewear and Optical
 |
New AR Coating Improves Contrast Sensitivity
A common complaint from spectacle wearers—and whoever may be talking to them—is the distracting reflections caused by light rays “bouncing off” the lenses of their glasses. Thankfully, lens manufacturers have long used anti-reflective coatings to reduce reflections and improve overall visual quality. One such company, Shamir Optical, recently launched a new AR coating called Glacier Expression that it touts as something even better than existing products.
According to Shamir, people wearing glasses coated with Glacier Expression demonstrated a 25-percent increase in contrast sensitivity vs. standard AR coating (no brand was specified), leading to improved reaction times during daily activities such as night driving. The company says the new coating also improves how wearers look to others—thereby improving person-to-person connection—and how their eyes feel, by reducing eyestrain.
In addition, Shamir Insight just announced an update to its Autograph Intelligence progressive lens design that the company says better reflects real-world visual needs, particularly during computer use. Visit https://shamir.com/us.
Stay on Top of Patients’ Vision Plans
If you’ve got an optical shop in your office, First Insight has coupled its EHR system MaximEyes with GPN’s EdgePro financial analysis software to help you keep better track of your business. First Insight says this new integration provides eye-care professionals with “real-time practice analytics that will empower doctors and staff to learn more about their business while maximizing revenue.”
With the EDGEPro and MaximEyes integration, doctors and staff use an application programming interface that automatically pulls data from the practice management system into EDGEPro nightly, so it’s ready when they need it, the company says. First Insight says, “EDGEPro lets you customize dashboards, find lost opportunities, manage frame boards, analyze managed vision care (MVC) plans and track staff performance.”
For information, visit https://www.first-insight.com/blog/edgepro-practice-analytics-maximeyes-empowers-eyecare/.
Testing and Planning
Glaucoma- and Retina-specific Testing Protocols
Recently, M&S Technologies announced a roll-out of six new enhancements for their Clinical Trial Suite (CTS), the DVA-5000, that the company says specifically address the needs of glaucoma and retina trial sponsors and researchers. The company claims that these automated testing protocols are more efficient and accurate than traditional testing methods.
M&S’ CTS modules already feature multiple visual acuity and contrast sensitivity function testing algorithms, all of which adhere to the ANSI and ISO standards and are recognized by the Food and Drug Administration for all clinical trial phases, including PMA trials. The company says that using their testing protocols can save trial sponsors and researchers significant time, as well as reduce the risk of erroneous data due to human error by calculating results automatically. CTS test results are available as a letter score, logMAR acuity notation, decimal score and Snellen equivalent. The company says that secure reports are immediately available in XML or CSV format to export to a reading center or other location.
For information, visit mstech-eyes.com.
LKC Technologies Launches Enhanced PhNR Protocol
LKC has launched a protocol enhancement for the RETeval, a handheld, full-field, non-mydriatic ERG testing device, that the company says helps hone the accuracy of the machine.
LKC says the new algorithm provides a 4-times improvement in test/retest variability, and a normative reference range that’s 1.7-times narrower. The result is a “more sensitive tool aiding in the detection of abnormal ganglion cell function,” the company states.
The company says the device is easy-to-use and comes with a large reference data set for straightforward interpretation. It requires no corneal contact, dilation or anesthetic for most tests, including this new PhNR protocol, LKC says.
For information, visit https://lkc.com/products/reteval/.



