The Technology
Though the Light Adjustable Lens has been available in Europe and Mexico since 2008, information about the lens and interest in it the United States have waxed and waned for more than a decade, as it made its way through various studies, so it probably helps to have a refresher on how the lens works:
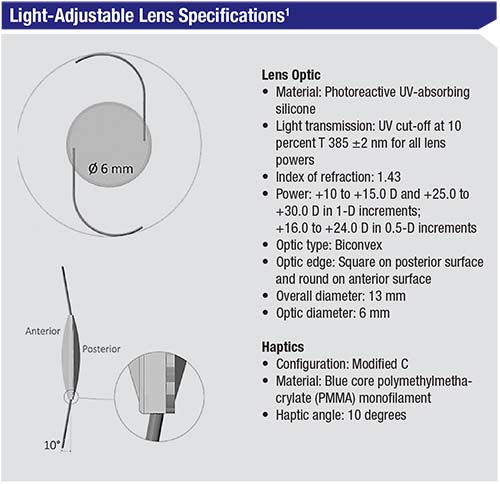
The RxLAL is a three-piece, foldable lens with a squared posterior optic edge; it’s made out of “photoreactive silicone.” The last part of the description is key to the
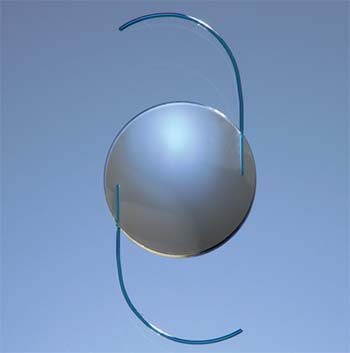 |
Sioux Falls, South Dakota, surgeon Vance Thompson, who participated in the study, explains the adjustment and lock-in process further. After the UV light spatially polymerizes the photoreactive silicone, over the next two days, through the process of diffusion the lens changes shape, he says. “So, if you want an increase in curvature, you illuminate the central portion of the lens, polymerizing more of the material,” he explains. “Then, there will be an increase in thickness in that area. It does this very specifically, with very specific mathematics. If you want to take away power, you would expose the peripheral part of the optic, and the unpolymerized material would diffuse to that area. This causes an increase in the peripheral curvature and takes away power from the optic. This can be done in a toric fashion, too—basically in whatever pattern you can describe mathematically. You have your shape change after two days, at which point you can schedule patients for the lock-in, which we did at three days postop in the clinical trial. At that three-day postop point, you can do the lock-in or another adjustment, if necessary [two adjustments were allowed in the trial]. The lock-in step involves illuminating the entire lens. After the lock-in, the whole lens is polymerized.”
To decrease the chances of ambient UV light affecting the lens before the lock-in step, the protocol requires the patients to wear UV-blocking glasses, mostly while outdoors. “We had zero issues with patients complying with that requirement,” Dr. Thompson says. “When you tell a patient that, for the first time ever, we can customize the implant in his eye to his visual needs, but in order to do that we need a little bit of healing, and a little bit of light adjustment until we fully lock in the lens, and he needs to protect his eyes from the sun, the patient usually says that it’s no problem.”
Lens Performance
In the FDA study of the lens, 390 eyes were implanted with the RxLAL, while 195 received a traditional monofocal lens and acted as a control group. The RxLAL group was actually targeted for slight hyperopia.
“The FDA wanted to see if the technology actually worked,” says Kevin Miller, MD, professor and chair of ophthalmology at UCLA’s David Geffen School of Medicine. Dr. Miller also participated in the RxLAL trial. “In the clinical trial, we were forced to leave the patients with some hyperopia—+0.5 or +0.75 D or so—because it’s easier to take them in the myopic direction than the hyperopic direction. So, the patient’s impression of his vision in the first three weeks would be, ‘It’s OK, but it’s not great.’ Then, when we’d do the first adjustment, three or five days later they’d say, ‘Whoa! My vision’s so much better!’”
Citing the study, Dr. Thompson says that 99.7 percent of the patients saw 20/40 or better uncorrected postop, and 91.6 percent were 20/25 without glasses. “Getting 91.6 percent of patients to 20/25 or better has never happened before in cataract surgery in any FDA-monitored study,” Dr. Thompson avers. “These are LASIK-like results.
“After the adjustment, 92 percent of the patients were within 0.5 D of intended, and 99.5 percent were within 1 D,” Dr. Thompson continues. “On average, there were 1.65 adjustments required. If I wanted to perform postop PRK or LASIK, in comparison, I’d need to wait three months for the wound to stabilize. With this, since we’re adjusting the power of the optic, we can start at three weeks postop.”
In terms of the toric adjustment, 82 percent of subjects had no more than 0.5 D of astigmatism at six months, and 98.5 percent had no more than 1 D. There were zero postop rotations due to axis misalignment. “It’s extremely stable,” Dr. Thompson says. “Even though other lenses can rotate in the early postop period, they’re typically stable by three weeks due to capsular contraction. But with this, we’re not adjusting it until the three-week mark, so it’s not only very stable, it’s locked in [place].”
The lens
 |
“The worst thing I experienced in the study was a patient with a macular burn,” Dr. Miller continues. “It happened because they had changed the supplier of the UV filter for the Light Delivery Device and the new filter was out of spec. The eye got exposed to more light than it was supposed to. The vision dropped to 20/150, but the patient recovered to 20/22 vision.” Some other adverse events that emerged in the FDA review include 1.7 percent of patients who needed a secondary surgical intervention, which was significantly higher than the historical rate (0.5 percent). These SSIs consisted of the following:
• explant due to the faulty UV filter mentioned earlier;
• explant due to a scratch on the LAL optic that occurred during implantation; lens replacement was required three weeks postop, and the secondary procedure had complications (final BSCVA: 20/20);
• explantation because the subject requested lens replacement prior to light treatment (final BSCVA: 20/15);
• Descemet’s stripping endothelial keratoplasty due to corneal edema caused by implantation problems (final BSCVA: 20/26.4);
• two incidences of lysing of iris adhesions (one accompanied by a sphincterotomy) to treat posterior synechiae that were limiting pupil dilation (final BSCVA: 20/17.4 in both instances); and
• barrier laser treatment for hemorrhagic posterior vitreous detachment and a horseshoe retinal tear with subretinal fluid nine months postop (final BSCVA: 20/14.5).1
At 12 months, one eye in the RxLAL group and four in the control group had a decrease of two or more lines of BSCVA.
A couple of adverse events unique to the RxLAL’s UV-light adjustment process were also investigated, namely red-tinted vision (erythropsia) and color vision abnormalities. The highest rate of erythropsia occurred prior to the second lock-in treatment, with 49 percent of patients reporting mild erythropsia. This decreased to 17.7 percent at one week after that lock-in, and to 0.5 percent at six months. One patient (0.3 percent) had mild erythropsia at the 12-month exam, but it resolved two months later.1
As for color vision, seven RxLAL (1.8 percent) eyes had a new tritan anomaly (difficulty distinguishing between blue, violet and green). Five resolved after the adjustments were complete, but two persisted. One of the latter two was due to the faulty UV filter, and still had the tritan anomaly four years later. The researchers note that both of the persistent and all but one of the transient tritan anomalies occurred before a certain safety improvement was made to the LDD to reduce the amount of UV exposure.1
Putting It into Practice
Though the company hasn’t specified a date for the rollout of the lens, surgeons have ideas how it might be used in practice.
“For patients, I think the benefit is they don’t have to make a decision about the type of vision they want preoperatively,” muses Dr. Thompson. “Unlike fitting glasses, with cataract surgery we can’t show them exactly what the result will be if we correct their dominant eye to plano and the non-dominant to -1 D, because they have a cataract—their vision is blurry. With this lens, though, for the first time we can show them their visual options after the surgery and they can choose where they want their optical power to be. You can start discussing ‘optimized monovision’ where you can change the power of the implant so that one eye is set for the amount of monovision they need for reading or the distance they want to work at.”
Dr. Miller envisions this, as well. “You can play with it,” he says. “You can ask, ‘Do you prefer distance vision and vision at computer distance? A little farther out? A little closer? You could simulate it with a contact lens and then dial that amount into the IOL. Though the ‘screaming need’ is for some sort of multifocal correction for distance and near vision, this lens won’t provide that just yet. However, the company had to get the base platform onto the market first.
“The initial interest from surgeons will be for treating post-refractive surgery eyes, which will be off-label,” Dr. Miller continues. “The study didn’t enroll those types of eyes, but you can do them. These are the types of patients that we surgeons obsess over. They’re particular about their refractive outcomes, but we have a hard time delivering good outcomes for them. So, everyone wants a solution. Therefore, my guess is that surgeons’ initial foray into this technology will be for treating these eyes. Then, when they see that it works, they’ll expand the LAL’s use to regular eyes, in a way similar to how toric IOLs were first used for the high astigmats and then their use filtered down to the less-challenging eyes.” REVIEW
Drs. Thompson and Miller consult for RxSight.
1. FDA.gov. LAL Summary of Safety and Effectiveness. https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160055B.pdf. Accessed 11 December 2017.