Today, visual fields are commonly used to help diagnose and monitor glaucoma. Here, several experienced clinicians share strategies that can help maximize the effectiveness of this technology.
Evaluating SAP Visual Fields
Elliott M. Kanner, MD, PhD, assistant professor of ophthalmology at the Hamilton Eye Institute,
Although there are standard indices of reliability for automated white-on-white perimetry and specific visual field patterns that suggest poor reliability (such as a cloverleaf), these fields can still be difficult to interpret. To evaluate these patients using a visual field, it's important to do four things:
• Determine whether the visual field deficit is consistent with a glaucomatous pattern. The patterns of visual field deficits for glaucoma are typically described as nasal steps, arcuate deficits and temporal wedges. (See examples, below.) Other recognized patterns include paracentral deficits and overall decreased signal (which is more subtle and less reliable). Patterns of loss that respect vertical meridians are less likely to indicate glaucomatous loss.
• Determine whether the deficit is consistent with optic nerve findings. Careful examination of the optic nerve will often reveal thinning of the tissue in the areas corresponding to the visual field loss, or a general decrease in tissue for overall decrease in sensitivity. This suggests that the deficit may indeed reflect glaucomatous damage. You can also check the nerve fiber layer thickness using instruments such as the HRT or GDx. In many ways they provide information similar to that obtained by direct observation of the optic nerve, and may help in cases where the nerve is difficult to assess, such as in a high myope.
• Determine whether the deficit is consistent with the patient's visual function status. A large complete central deficit in the visual field in a patient with good acuity could indicate that the deficit is a testing artifact. Large areas of peripheral loss can be confirmed with confrontational testing, or by having the patient describe any areas of loss that he or she is aware of.
• Determine whether the deficit is consistent with other testing. In cases of asymmetric visual field loss of questionable reliability, a corresponding afferent pupillary defect can be very strong supporting sign.
Although SAP visual fields are imperfect, interpreted in combination with careful examination and optic nerve measurements they can usually allow you to paint a fairly accurate picture of the status of the optic nerve.
Practical Testing Tips
Philip P. Chen, MD, associate professor and acting chairman at the department of ophthalmology at the
To get the most accurate data from perimetry testing, it's helpful to have both the technician and doctor remember to do a few basic but important things. For example, your technician should:
• Make sure the patient is comfortable. If the patient isn't, he may move in the middle of the test, possibly affecting the validity of the results. The technician should actively check this by asking the patient, "Is this a position you can sit in for the next five to 10 minutes?"
• Make sure the patient knows when the test is starting. If a patient takes a while to realize the test has started, the result is a strange-looking visual field that resembles a reverse cloverleaf. (See illustration, below.) Alerting the patient can save the time and effort of a wasted test.
• Check the foveal threshold. This is a short test option that takes about 15 seconds that can be employed at the beginning of the primary test if you're using the Humphrey perimeter (Carl Zeiss Meditec). It can provide valuable information, for example, when a patient with advanced glaucoma that is close to fixation complains that her vision is worse, yet Snellen acuity and the 24-2 field appear unchanged. Some practices prefer to save time and not run it, but it can be very worthwhile.
One important caveat: The patient focuses on a different, non-central fixation point for this test. So, once the foveal test is complete, the technician must remind the patient to switch his fixation back to the central point. Otherwise, the primary test results will be shifted down by 10 degrees.
• Remind patients they can pause the test. Sometimes when I've encountered a test result that looks poor, the patient has said, "My other eye was watering underneath this patch, and I couldn't concentrate." Your technician should make sure the patient knows how to pause the test if a problem like this arises.
• Encourage patients to speak up if something's wrong. If possible, have the technician stay in the room during the test to respond if a problem arises. If you don't want to keep the technician in the room, make sure he stays near enough to notice if a patient needs assistance.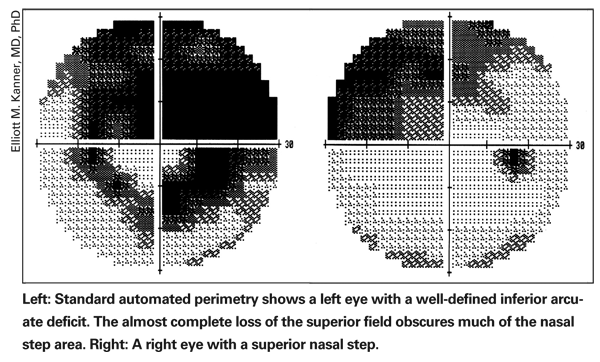
When interpreting the results, the doctor can also prevent errors and wasted time by keeping an eye out for inadvertent technician errors. (These are rare in most practices, but everyone is human.)
• Scan the patient and test information on the printout. To avoid unnecessary surprises:
— Make sure the correct name is on the printout.
— Confirm that the technician has listed the correct date of birth. The wrong year can affect the software's interpretation of the results, since it uses an age-matched normative database for comparison.
— Make sure the correct refraction was used for the test.
— Make sure the test strategy/algorithm used was the same as in previous tests; different versions of a test are not necessarily comparable. For example, if you've been using SITA standard and then you use SITA fast, it may look as if the patient improved dramatically. Conversely, if you've inadvertently switched from SITA fast to SITA standard, the test results will look worse, and you might conclude that the patient is progressing.
— Make sure the stimulus size was not changed. Although most tests are done with a size-3 stimulus, you'll occasionally have patients with advanced damage who need to be tested with a size-5 stimulus. If a patient's been followed using a size-5 stimulus all along, and one day the technician tests the patient with a size-3 stimulus, the results will make it appear that the patient has gotten much worse.
Detecting Progression with SAP
Angelo P. Tanna, MD, assistant professor of ophthalmology and director of the Glaucoma Service at the Feinberg School of Medicine at Northwestern University, offers these suggestions for using SAP to help monitor glaucoma progression:
With the currently available technology routinely used in clinical care, there is evidence that the development of structural glaucoma damage usually occurs prior to the development of functional damage. Nevertheless, clinical trials such as the Early Manifest Glaucoma Trial have shown that in patients with pre-existing glaucomatous visual field loss, further progression usually manifests itself as visual field progression only. Hence, one of the most important clinical uses for perimetry is the detection of progressive damage in patients with established glaucoma.
Unfortunately, detection of progressive visual field loss in eyes with glaucoma is a complex problem for which there is no gold-standard solution. The most important obstacle to detecting progressive visual field loss with SAP is that visual sensitivity at various locations in the visual field fluctuates, both during a single visual field test (short-term fluctuation) and over the course of repeat testing from day to day (long-term fluctuation). This is true even in patients without any ocular disease process, although the magnitude of fluctuation is greater in patients with glaucoma.
Many factors correlate with the magnitude of anticipated fluctuation in visual sensitivity. The two most important factors are: 1) the distance of a testing location from fixation (the farther it is from fixation, the greater the degree of anticipated fluctuation); and 2) the severity of damage at that location. (In damaged areas of the visual field the magnitude of this fluctuation increases as a function of the severity of the baseline visual field loss.) For these reasons, a large magnitude decline in threshold sensitivity may not represent true glaucomatous progression, especially if the point in question is far from fixation and/or severely damaged at baseline.
To help surmount these obstacles, one of the most important steps in the early management of a newly diagnosed glaucoma patient is to establish a reliable baseline set of visual fields against which future comparisons can be made. To do this, obtain at least two reliable baseline visual fields soon after diagnosis. (Note: Tests obtained during the period when the patient is scaling the steep portion of the testing learning curve should not be included among the baseline visual fields to be used for future comparison.)
Also, because fluctuation in visual sensitivity is a confounding factor, if a follow-up visual field test discloses progression, it must be repeated at least once—preferably twice—before you conclude that the deterioration you find is indicative of true progression. Most clinicians know from experience that patients often exhibit apparent visual field progression on a single test, only to have the next test clearly demonstrate stability relative to the baseline visual fields, and multiple clinical trials have confirmed this. This may be the case even when worsening of visual sensitivity exceeds the anticipated magnitude of fluctuation at several locations in the visual field.
Progression: Analyzing the Data
Most clinicians evaluate serial visual fields without the aid of computer software. Physicians simply "eyeball" multiple pages of visual fields to look for signs of deterioration. This is time-consuming and probably less sensitive and specific for the detection of progression than other more sophisticated techniques. 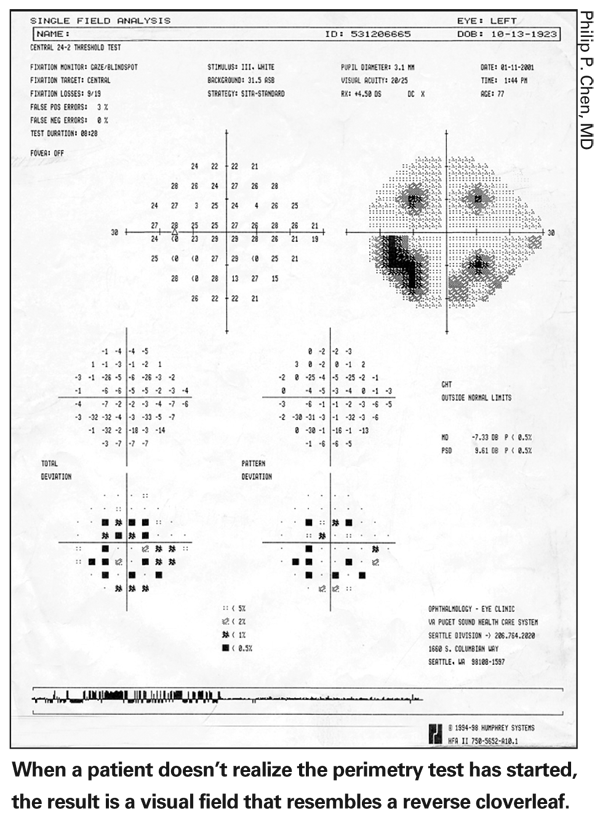
Because of the numerical data involved in visual field testing, serial visual fields are particularly amenable to statistical analysis for the detection of progression. There are two primary methods for doing this:
• Trend-type analysis. This uses linear regression analysis to determine if the data are showing a tendency toward declining threshold sensitivity over time. Linear regression analysis can be used to evaluate a global index value such as the mean deviation on the Humphrey Field Analyzer, or it can evaluate each individual location in the visual field (i.e., pointwise linear regression analysis). The latter can be done with Progressor software, which was developed at Moorfield's Hospital in
• Event-type analysis. For example, you might define a 2-dB decline in the mean deviation as constituting progression. The problem with this approach is that progressive cataract can cause this same kind of worsening of the mean deviation.
In the Early Manifest Glaucoma Trial, investigators defined visual field progression as deterioration that exceeded the anticipated magnitude of fluctuation at the same three points in the visual field on three consecutive visual field testing sessions. (The three progressed points were not required to be contiguous.) The data from the pattern deviation maps were used to judge progression because those values are relatively resistant to the impact of progressive cataract.
Using patients identified during the EMGT, a database of hundreds of glaucoma patients who underwent perimetry four times over the course of one month was created. This information was used to establish the range of fluctuation in visual sensitivity that could be expected to occur in stable glaucoma patients at different locations of the visual field with varying degrees of severity of damage at baseline. Once those data were available, the values from each follow-up visual field were compared with the mean values from two baseline visual fields for subjects participating in the EMGT. (Commercially available software called Glaucoma Progression Analysis uses this methodology.)
So, if you use perimetry as a means to detect glaucoma progression before progressive structural damage is evident, be sure to obtain at least two reliable baseline visual fields as soon as possible after diagnosis. If a large magnitude decline in threshold sensitivity later suggests that progression is occurring, be sure to repeat the test at least once—preferably twice—and use appropriate statistical analysis to confirm that progression has occurred. (The new progression analysis software from Humphrey automates this process, making it very useful for clinicians managing large numbers of patients.) Also, be sure to look at the entire amalgam of clinical information before drawing any conclusions about whether progression has occurred
Using SWAP and FDT
Sarwat Salim
Standard automated perimetry is the most common form of visual field testing performed in glaucoma practices. However, it's important to know that because of considerable redundancy in neural pathways and the nonselective nature of conventional perimetry, SAP may not fully reveal underlying neuronal damage. In fact, histological evidence has shown that a significant number of ganglion cells may be lost before visual deficits are manifested on SAP.
One way to improve detection of early functional loss is to target a specific subset of ganglion cells that have sparse distribution. This rationale led to the development of SWAP and FDT. In SWAP, the koniocellular pathway, which is involved in transmission of the short blue wavelength, is activated by using a blue stimulus on a yellow background. This method of testing has been shown to detect visual field loss up to five years earlier than SAP.
Similarly, FDT is based on the concept that a combination of low spatial frequency and high temporal frequency preferentially targets ganglion cells of the magnocellular pathway. These cells are believed to be primarily involved in the detection of motion and flicker. FDT presents the subject with the frequency doubling illusion by counterphasing a low spatial frequency sinusoidal grating with a high temporal frequency. This has been shown to have high sensitivity and specificity for early detection of glaucoma and may be predictive of later losses, given its low test-retest variability compared to SAP.
A few strategies for getting the most value from these instruments:
• When using SWAP, interpret results with caution when the patient has coexisting cataracts. SWAP is subject to higher test-retest variability than SAP and is more influenced by media opacities. So in this situation, place more emphasis on the glaucoma hemifield test and the pattern deviation plot. These are more sensitive indicators of localized glaucomatous loss and are less affected by generalized reduction in signal.
• Consider using FDT for patient screening. Although FDT can be used for both threshold and screening tests, the instrument is portable, inexpensive and provides results quickly; the screening test only takes 45 to 90 seconds per eye. There is also some evidence that this device is more tolerant to refractive error than other perimetric tests. These characteristics make it especially useful for screening. (The Humphrey Matrix Perimeter with Welch Allyn FDT uses frequency-doubled perimetry to map additional visual field points. This instrument is currently under evaluation for clinical use.)
• Don't rely on only one of these instruments for a definitive diagnosis. These instruments should be seen as complementary.
• Interpret the results in the context of the patient's overall clinical picture. Pay attention to other risk factors such as race, family history, and corneal thickness, the optic nerve status, and the magnitude and fluctuations of the intraocular pressures.




