Though we know a good deal about corneal marginal infiltrates, other aspects of the problem remain a mystery. We know that infiltrates can be caused by either an infectious or non-infectious (sterile) condition, the latter being associated with contact lens wear, bacterial toxins, post-surgical trauma, autoimmune disease and other toxic stimuli. The pathogenesis of sterile marginal infiltrates, however, is not yet completely understood. In this article, we'll explore what we know about this enigmatic condition and how to manage it if you encounter it in your practice.
• Signs of infiltrates. The natural course of events following injury to the corneal surface involves a cascade of inflammatory and immune cells that arrive on the scene to repair any damage. The corneal tissues are normally devoid of blood and lymph vessels, thus protection is provided by mediators from the conjunctiva and tear film. The presence of white blood cells in the peripheral cornea is referred to as an infiltrate.
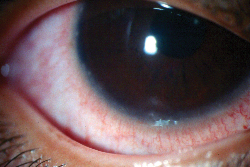 |
| Marginal infiltrates usually appear as distinct white dots along the corneal periphery, with conjunctival hyperemia. |
Sterile marginal corneal infiltrates appear as small (£ 1mm), gray-white circumlimbal lesions. Separated from the limbus by about 1 mm of clear space, they may be accompanied by epithelial defects. It's more common for infiltrates to appear individually, but they can also appear in groups and are sometimes bilateral.1 Patients with sterile marginal infiltrates may occasionally be asymptomatic, but commonly present with symptoms of mild quadrantic conjunctival hyperemia, little or no chemosis, mild ocular irritation and normal vision. Larger, central infiltrates associated with photophobia, discharge, pain and a deeper redness are often infectious and should be cultured and treated accordingly.2
The pathogenesis of sterile marginal infiltrates appears to involve an acute immune response to corneal damage. The introduction of antigen to the corneal surface can release inflammatory mediators to the periphery, which triggers vasodilation. White blood cells stream into the cornea and coalesce to form an infiltrate. These cells can release proteases and inflammatory mediators that cause epithelial breakdown, which appears as overlying staining. After an infiltrate has healed, a corneal scar may form. A frond of blood vessels is left, extending beyond the normal vessel range, accompanied by a slight haze.3
• Infiltrates and contacts. The first reports of sterile corneal infiltrates appeared more than 25 years ago, and were tied to the wearing of soft contact lenses.4 Contact lens-associated corneal infiltrates have been correlated with the presence of bacteria, extended lens wear, noncompliance to lens replacement or care schedules, and hypersensitivity to lens material or solution preservatives.5 Hypoxia, primarily as a result of overnight wear, has been linked to infiltrate formation, but appears to be associated only with a higher likelihood of central and infectious infiltrates.6 In general, contact lens use has been shown to induce a number of physiological and morphological changes in the cornea, which are manifested as cellular responses that can lead to the development of infiltrates.6 Damage to epithelial cells due to lens-associated trauma increases the release of inflammatory cytokines, leading to infiltrate formation. The lens itself can trap bacteria, which may also adhere more readily to damaged epithelium.
Even patients with sterile infiltrates often have positive cultures taken from lenses, lens cases, conjunctivae or eyelids. General lens colonization is common, however, in the presence or absence of infiltrates and the incidence of positive lens cultures is similar between symptomatic and asymptomatic patients, which further complicates the connection between bacteria and infiltrates.7,8 In these cases, infiltration is most likely the result of an immune response to bacterial toxins or the presence of bacteria in the form of biofilms, which are undetectable by conventional culture methods.9 Studies have shown that the Gram-negative bacterial cell- wall component, lipopolysaccharide (LPS), participates in the release of proinflammatory cytokines such as IL-1a, a potent chemoattractant of neutrophils that aids in neutrophil degranulation, inducing an allergic response and subsequent infiltrate formation.10
• Staph infection. The bacterial enigma presents itself in the case of staphylococcal blepharitis, as well. Studies have long shown correlations between marginal infiltrates and bacteria such as Staphylococcus aureus on the cornea.11,12 However, lesion cultures associated with the presence of S. aureus are commonly negative. It is theorized that staphylococcal cell-wall antigens such as endotoxin B or ribitol teichoic acid trigger a hypersensitivity reaction. Prolonged topical ocular administration of S. aureus in rabbits produced a higher number of teichoic acid antibodies (IgA and IgG) and increased the subsequent development of corneal infiltrates compared to rabbits that did not receive topical S. aureus. In addition, repeated S. aureus administration intensified blepharitis and related infiltrates only in animals immunized with inactive S. aureus, indicating that the lesions were sterile.13
| Is Therapy Best for Self-Limiting Conditions? |
| Sterile peripheral marginal infiltrates are indeed a self-limiting condition, much like a cold or headache. Without treatment, they disappear within a week or two most of the time. Infiltrates normally cause only mild symptoms and very rarely progress to something more serious. However, patients who present with symptoms such as ocular redness, tearing, or discomfort benefit from topical steroid therapy in terms of an accelerated improvement in quality of life. The same is true for patients who take decongestants for a cold or pain relievers for a headache. Drug treatment allows for quicker relief from uncomfortable symptoms. Yet, is this enough rationale for therapy in the category of self-limiting conditions? There is debate over whether or not therapy for marginal infiltrates is warranted. Clinicians must decide what level of risk is worthy of prophylaxis. Remember, a diagnosis isn't perfect all the time. Infiltrates accompanied by epithelial breakdowns provide easy entry for bacteria, which leads to ulcer formation in a small percentage of cases. Is the likelihood of such an event rare enough that any risk can be willingly accepted? If not, should there be a standard of care to cover all bases for the condition of sterile marginal infiltrates? These questions are of moral and ethical significance. Perhaps it should be an individual decision made by the clinician and patient. The concerns of the patient certainly play an important role. If the worst-case scenario is a consequence you couldn't live with, you'll want to protect against the cause. Why else do people buy insurance? —Mark B. Abelson, MD |
• Allergy and infiltrates. In addition to bacterial toxins, antigens associated with vernal keratoconjunctivitis and drugs that act as allergens have been implicated in the development of marginal infiltrates through corneal involvement in the allergic response pathway. Long-term sensitization of antigen on the corneal surface is believed to initiate a biphasic response consisting of type-1 and type-4 hypersensitivity reactions. Antibody exposure to the antigen initiates type-1 hypersensitivity, an IgE-mediated reaction leading to mast-cell degranulation and histamine release. Repeated exposure, in the case of VKC, instigates late-phase hypersensitivity, which increases the transport of polymorphonuclear leukocytes into the epithelium that may ultimately cohere to form a marginal infiltrate. In VKC, most of the mediators involved in this process are found in increased amounts.14
• Anesthetics. Sterile marginal infiltrates have also been linked with various instances of compromised corneal epithelium such as that associated with the use of topical anesthetics. These agents have been associated with the development of sterile corneal ring infiltrates resulting in corneal scarring and vision loss. The mechanism of the anesthetic's toxicity is unknown, but it is theorized that there is either a direct toxic effect to the corneal epithelium or impairment of the trophic action of corneal nerve fibers.15 Epithelial breakdowns also seem to be pivotal in triggering the inflammatory reaction responsible for the development of corneal stromal infiltrates in patients with recurrent corneal erosions, perhaps by creating a portal of entry for antigen.
• Refractive surgery. More recently, sterile marginal infiltrates have been associated with ocular trauma resulting from PRK and LASIK surgery. Corneal infiltrates accompanied by pain, photophobia and injection have been reported one to three days post-PRK, usually resulting in corneal scarring and loss of vision. Cases of PRK-induced infiltrates were first reported when non-steroidal inflammatory drugs, in conjunction with an occlusive soft contact lens, were substituted for the conventional bandage occlusion following surgery. The condition occurs in an estimated one of 300 cases and is believed to arise mainly due to the complications of contact lens use.17 Sterile peripheral corneal infiltrates have also been reported following LASIK surgery; they resolved without complications and appear to be immunogenic in origin, though they're poorly characterized.18
• Autoimmune conditions. Research has also shown corneal infiltrates to be part of the symptom spectra of various systemic autoimmune diseases including rheumatoid arthritis, histiocytosis, paraproteinemia, polyarteritis nodosa, Crohn's disease and Wegener's granulomatosis. Ocular diseases such as Mooren's ulcer, Cogan's syndrome and vernal keratitis are also associated with the appearance of marginal infiltrates, though these diseases are often differential diagnoses. Patients with a history of dry eye, rosacea or a vitamin A deficiency may present with marginal infiltrates, though the etiological connection is uncertain.
Treatment
Left untreated, marginal infiltrates generally disappear within a week or two. Ocular steroids have been shown to be the best and only recognized drug therapy for sterile marginal infiltrates, and their application will shorten the course of inflammation, regardless of causative origin. For many patients, a quicker recovery from symptoms such as redness, tearing, and discomfort is important for improving their quality of life. Steroids are often prescribed in conjunction with an antibiotic in order to decrease the chance of developing a secondary infection or corneal ulcer and to protect against misdiagnosis. Use of steroid treatment should be carefully monitored at the lowest dosage necessary to control inflammation.
If the infiltrate is of unknown origin, it's advisable to take a culture and start a combined treatment of a steroid and broad-spectrum antibiotic like a fluoroquinolone. Infiltrates suspected of being infectious or those that present as part of a more serious ulcerative condition should be treated as soon as possible in order to prevent further damage.
In the case of contact lens-associated infiltrates, lens wear should be suspended until infiltrates heal. Prevention of infiltration can be promoted with the elimination of various mechanical and toxic stimuli that have been shown to induce the development of marginal infiltrates. Improved eyelid hygiene, a change in lens design, or a change in wearing schedule may be necessary.
Sterile marginal corneal infiltrates can stem from an assortment of ocular and systemic etiologies. Typically, the initial stimulus is damage to the cornea, whether the origins are immunologically mediated, traumatic or toxic. The presence of an infiltrate and the signs and symptoms associated with it are a result of the subsequent cascade of inflammatory and immune reactions.
Topical use of steroids impedes the progression of these reactions and speeds resolution of the condition, and drug treatment in conjunction with elimination of the initial stimulus will promote healing. Most important, an improved understanding of the pathogenesis of infiltrate formation will provide a basis for managing this mysterious malady in the future.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals. Ms. Plumer is a research associate at Ophthalmic Research Associates in North Andover.
1. Donshik PC. Editorial: Peripheral corneal infiltrates and contact lens wear. CLAO J 1998; 24:3:134,136.
2. Poggio EC, Abelson MB. Complications and symptoms with disposable daily wear contact lenses and conventional soft daily wear contact lenses. CLAO J 1993;19:95.
3. Bazan NG, Bazan HP. Ocular responses to inflammation and the triggering of wound healing: lipid mediators, proto-oncogenes, gene expression, and neuromodulation. In: Bazan NG, ed. Lipid Mediators in Eye Inflammation. Braquet P, ed. New Trends in Lipid Mediators Research, 5th ed. Basel: Karger 1990:168-80.
4. Berstein HN, Lemp MA. An unusual keratoconjunctivitis occurring after longtime wearing of the AO Softcon (formerly Griffin or Bionite) hydrophilic contact lens. Ann Ophthalmol 1975;7:97-106.
5. Robboy MW, Comstock TL, Kalsow CM. Contact lens-associated corneal infiltrates. Eye & Contact Lens 2003;29:3:146-154.
6. Liesegang TJ. Physiological changes of the cornea with contact lens wear. CLAO J 2002;28:12-27.
7. Sankaridurg PR, Sharma S, Wilcox M, et al. Colonization of hydrogel lenses with Streptococcus pneumoniae: Risk of development of corneal infiltrates. Cornea 1999;18:289-295.
8. Corrigan KM, Harmis NY, Willcox MDP. Association of Acinetobacter species with contact lens-induced adverse responses. Cornea 2001;20:463-466.
9. Elder MJ, Stapleton F, Evans E, et al. Biofilm-related infections in ophthalmology. Eye 1995;9:102-109.
10. Schaefer K, Abelson MB, Richard KP, Schultz CL. Ulceration of New Zealand white rabbit corneas induced by intrastromal injection of IL-1. Invest Ophthal Vis Sci 1995; 36/Suppl:4668.
11. Mondino BJ. Inflammatory diseases of the peripheral cornea. Ophthalmol 1998;95:463.
12. Mondino BJ, Lahedi AK, Adamu SA. Ocular immunity to Staphylococcus aureus. Invest Ophthalmol Vis Sci 1987;28:560.
13. Schultz CL, Morck DW, McKay SG, et al. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res 1997;64:3-9.
14. Abelson MB, Slugg AP. Marginal infiltrates in the cornea. Allergic Diseases of the Eye. W.B. Saunders Co, Philadelphia, PA. 2000:162-3.
15. Maurice DM, Singh T. The absence of corneal toxicity with low-level topical anesthesis. Am J Ophthalmol 1985;99:6:691-696.
16. Tabery HM. Corneal stromal infiltrates in patients with recurrent erosions. Acta Ophthalmol Scand 1998; 76:589-592.
17. Teal P, Breslin C, Arshinoff S, Edmison D. Corneal subepithelial infiltrates following excimer laser photorefractive keratectomy. J Cataract Refract Surg 1995;21:516-518.
18. Yu EY et al. Bilateral peripheral corneal infiltrates after simultaneous myopic laser in situ keratomileusis. J Cataract Refract Surg 2002;28:5:891-4.



