The same microkeratome or femtosecond laser that gives a refractive surgery patient a smooth corneal bed can do the same for lamellar keratoplasty patients, offering the potential for better vision postop than manual dissection. In this article, anterior segment surgeons explain the benefits this procedure offers, as well as offer tips for getting the best results with your grafts.
Indications
The technique is straightforward, using a microkeratome to both excise corneal pathology by cutting it out as a free cap, and also to cut a donor cap from an eye-bank eye.
According to Lee Wiley, MD, associate professor of ophthalmology at West Virginia University, the main indications for automated lamellar therapeutic keratoplasty are either to correct optical or tectonic corneal problems.
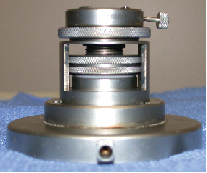 |
| Moria's Artificial Anterior Chamber is key to cutting the donor cornea. The donor corneo-scleral tissue is mechanically gripped at the top of the device and pressurized with IV BSS to ensure an even microkeratome cut. All images: Lee Wiley, MD. |
In the optical patients, they have an opacification or scar, usually confined to the top 200 µm of the cornea, that is decreasing vision. "In the optical indications, we're trying to get a clear visual axis and the best corneal curvature we can," says Dr. Wiley. "It's good for severe pathology in the anterior half of the cornea in which you're willing to trade off some of the visual benefits of the full-thickness transplant for the trauma resistance and lack of rejection issues and graft replaceability of the lamellar procedure." He says the replaceability can be a bonus in patients with granular or lattice dystrophy in which their condition recurs. "If the disc scars again, I can flip the original disc of tissue out and replace it," he says.
The procedure can also be useful in postop refractive surgery patients with irregular astigmatism that have no option other than a corneal transplant or a hard contact lens.
In tectonic indications, the structural integrity of the globe has been compromised in some way, and the surgeon needs to augment the tissue. Dr. Wiley offers a current patient as an example. "I currently have a patient with a large pterygium that I have to resect," he explains. "I'm going to augment the corneal area of pterygium removal with an ALTK-prepared lenticle of tissue."
Patients in whom the procedure isn't as effective are those with depressed pathology. "If you have a scar with a depression and try to create the recipient bed with the microkeratome, you'll reproduce the depression," says Dr. Wiley. "If you visualize what's happening, you can see why this occurs. The applanating pressure's going to shove the depression up against the applanating plate. You're then going to run your microkeratome through. When you look again, you're still going to have the depression, albeit a bit smoother, but it will still, to a great extent, be reproduced in the bed."
The Technology
The main device besides the microkeratome that's necessary to perform automated lamellar therapeutic keratoplasty is an artificial anterior chamber. Units are available from Moria for $13,500 and from Bausch & Lomb, with each company's chamber being configured to work with its particular microkeratomes. There are also off-brand artificial chamber devices on the market for use with the Intralase.
 |
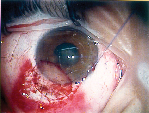 |
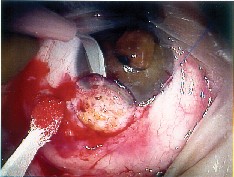
| In this keratoconus patient with a limbal dermoid, the surgeon removed the pathology manually, but then replaced it with a microkeratome-prepared graft. | |
The artificial chamber allows the donor tissue, which is just a cornea with a rim of sclera, to be pressurized enough to be cut. Since this device involves a considerable investment, thus far the procedure has been performed mainly in academic centers and corneal practices that receive a lot of referral patients.
Other key instruments are Colibri forceps for manipulating the corneal caps and a trephine system such as the Hanna or the UltraFit. The trephine system allows the surgeon to modify the size of the donor tissue to fit the recipient bed properly.
Graft Techniques and Tips
Surgeons are currently performing ALTK in two ways, according to Michael Taravella, associate professor at the University of Colorado. He has been cutting many donor corneal buttons to standardize his technique in preparation for his first full cases.
The first method involves cutting the recipient bed with the microkeratome, removing the pathology from the top 150-200 µm, and placing a donor cap of similar or slightly smaller diameter to replace the pathology that was removed.
The second technique proceeds in two stages. First, the surgeon cuts a large diameter flap in the recipient, measures the flap thickness to see how deeply he's cut, then lays the flap back down and waits a month or two for it to adhere properly. Dr. Wiley advises placing a bandage contact lens for the first night to ensure that lid disease doesn't pull up the flap. Then, after it's adhered, the surgeon uses a suction trephine that allows him to control the trephination depth to cut inside the flap diameter and peel back the recipient button to the depth of the original lamellar cut. He then uses the microkeratome to cut a cap from the donor cornea, and trephines a donor button from it. The donor button is then sutured onto the recipient bed. "The advantage with this method is the surgeon gets a more precise fit," says Dr. Taravella. "The disadvantage is he or she needs a larger corneal button in order to do it."
Dr. Wiley prefers the second method, because it allows him to match the diameter exactly, but he notes there are advantages to the overlay technique, too. "It's one step. Also, if you don't let the flap heal down long enough with the trephination technique, it could displace while you're sewing the button in place, causing the edges to lift."
• Cutting the donor. In the trephination method, after the recipient corneal cap has had time to adhere, the surgeon trephinates out the pathology, usually with a 7-7.5 mm trephine. Then the donor is prepared.
Surgeons say the donor cornea must have an adequate amount of sclera around its rim, usually about 3 mm or more, to allow the tissue to seat well in the artificial anterior chamber and stay that way throughout the microkeratome pass.
"You can't take a small rim and get away with it," says Dr. Wiley, "because it will herniate when you pressurize it and you'll get a very odd cut by causing a variation in the shape of the tissue that's presented to the blade." He also advises having a very even clamping force on the rim of the corneo-scleral tissue when you place it on the artificial chamber device so it can't slip when the chamber's pressurized.
The proper pressurization of the artificial chamber is a crucial step that needs to be done well if the procedure is to succeed, say surgeons.
"The way I achieve adequate pressure is to raise a BSS bottle with high-volume IV tubing 70 cm above the eye, and vent that with a bent 18-ga. needle so there's adequate venting into the bottle," explains Dr. Wiley. "I check it with a Barraquer tonometer after I've seated the donor tissue and tightened the ring to secure it. The pressure will be around 50-60 mmHg, maybe a little more."
He says the benefit of good pressurization comes when he makes the pass, and some fluid is displaced from the donor. "If tiny bits of fluid are displaced, you're still maintaining the pressure," he says. He doesn't like syringe techniques that use a small volume of BSS and try to build up the pressure with a syringe or pump. "With gravity-fed BSS, microleaks won't affect the pressure," he explains.
If the surgeon is doing an onlay technique, rather than trephinating a button, he has to match the donor cap to the bed he's created on the recipient. To help him do this, the Moria artificial anterior chamber has a height adjustment that raises or lowers the donor cornea against an applanation glass. The applanator has rings in a clear window that match up with the diameter of the cut, so the harder the cornea is pressed against the window, the wider the cut will be.
Dr. Wiley says this applanator can even be helpful to someone planning a trephination technique.
"Even when I'm not trying to match the diameter, it will tell you if your tissue is well-seated in the chamber," he says. "If you get an elliptical applanation, that means your tissue's herniated and you'll get an elliptical cut. It lets you assess the quality and diameter of the cut, so if it's circular on applanation, it will be circular when you cut it."
• Placing the donor button. Dr. Wiley cuts the donor to match the depth of the window in the recipient's cornea. "I oversize it, making it about 9 mm in diameter, then I take a punch that's 0.25 mm bigger than the recipient button that I removed and transfer it to the opening in the recipient cornea," he explains. He says that, at this point, you can maximize the visual outcome by taking a Mastel light or similar device and observing the corneal mire of the new button in the recipient bed.
"I rotate it in the bed until I have the best possible mire," he says. "I try to match the resection contour with the graft contour, and rotating it in the bed probably gives me the best chance of doing that. One of the issues we face in doing the procedure is that these flaps have shape, and, as we've learned from refractive surgery, when a surgeon makes a LASIK flap, it isn't a piece of tissue that's totally parallel to the cornea. It has a meniscus shape, and it also has a taper to it. That's why, when you remove a flap in LASIK, the visual quality isn't so good, because you've uncovered the shape you've induced with the keratome. So, in lamellar keratoplasty, we again are dealing with two things of different shapes fitting together. Therefore, by optimizing the position of the button in the bed, it's helped to eliminate some of the issues of irregular astigmatism."
• Deeper pathology. For pathology that's deeper than 200 µm, Dr. Wiley says the technique becomes, "somewhat questionable." For such eyes, he says it can help to have an excimer laser nearby ready to function in PTK mode.
"After you make the flap in the recipient, you can flip it back and add PTK pulses to clear any residual stromal deposits you may have missed when you created the flap," he says. "You just have to be careful not to go too deep."
• Suture technique. After the graft is oriented in the bed, Dr. Wiley places four cardinal stitches so the graft won't twist in place, and then places the running suture. He then removes the cardinal stitches and adjusts the running suture with a qualitative keratometer to make sure the suture tension is even and he has the best mires possible. He typically removes the suture after three to four weeks.
In some cases, he may place an imbrication stitch as first described by France's Georges Baikoff, MD. This suture involves not actually suturing the lenticle, but bringing a suture over from the limbus, bridging over the disc of tissue. He says this holds the graft in place with a suture that's tensioned from the periphery, so as not to induce any refractive change in the cornea.
• Results. The benefit that drew surgeons to ALTK in the first place as an alternative to traditional hand-dissection lamellar keratoplasty is the potential for better vision postop due to a smoother bed as created by the microkeratome, and the results show that. However, the visual results still aren't as good as penetrating keratoplasty's.
 |
 |
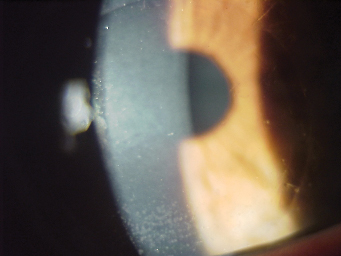
| A lattice dystrophy patient's vision preop was 20/100 (left). After ALTK with trephination of the pathology, the patient now sees 20/40 in the eye (right). | |
According to Dr. Wiley, ALTK will typically yield 20/40 to 20/60 vision with spectacles, with an occasional patient seeing 20/30. As to why the vision doesn't equal the level of PK, he thinks it has to do with the irregular astigmatism created in the matching of the graft with the bed. However, he says that these patients' postop vision can improve over time. "The longer you follow these grafts, the better they get," he says. "I've seen patients who were 20/100 right after their surgery come back a year or two later with 20/40 vision. I think this may be due to the epithelial remodeling that occurs when the tear film returns. The epithelium usually thickens greatly over any lamellar pathology or resection."
The Intralase Option
Though the femtosecond laser can efficiently make flaps that are very smooth and are of a very predictable depth, it's not always perfect when faced with an opacified cornea.
Karl Stonecipher, MD, of Greensboro, N.C., uses the Intralase in lamellar keratoplasty. "I think my laser-made flap gives me more of a predictable flap diameter because it's not as influenced by the K readings of the donor or the recipient, and is a little more precise in terms of flap thickness," he says. In his practice, he's found the Intralase cuts flaps with a variation of ± 14 µm in thickness.
"However, one of the disadvantages of lamellar grafts with Intralase is that if the patient has a dense scar, the Intralase has difficulty cutting through it," he says. "So, for this type of patient, you're more likely to use a mechanical cut on the recipient, but you can probably use the Intralase to cut the donor, because it will be clear." He also says he'd be more inclined to use a mechanical microkeratome on a patient with herpetic disease, because of the "inflammation associated with the laser."
Dr. Stonecipher is currently working on a study called the Keratobutton Project, which was started by two former employees of a North Carolina Eye Bank. The goal of the project is to cut numerous donor corneal buttons of various sizes, then preserve them in blister packages so surgeons in need of a graft, such as during a LASIK case that goes awry, can have quick access to donor tissue, similar to having IOLs of various powers on the shelf.
"We took a lot of donor corneas and cut buttons with the Intralase, then processed them so that they could be stored," Dr. Stonecipher explains. "Once they were processed, we placed them in a container and irradiated them with gamma radiation to preserve them. Then, we took them out and rehydrated them with BSS, which caused them to balloon to sizes much larger than the original cut. However, we've found that if we use Optisol to rehydrate them, they return to their original shapes."
The project is still ongoing, and Dr. Stonecipher and his colleagues are working to perfect such aspects of the process as achieving a smooth interface that is more optically neutral and preserving the tissue's glycosaminoglycans, which he feels are crucial to maintaining clarity when the graft is rehydrated.
Whether you use a microkeratome or a femtosecond laser, Dr. Wiley says that, if you have enough corneal patients to warrant doing ALTK, it is a very good procedure, adding, "The procedure is really wonderful when you need something to cover the cornea that tapers perfectly and is of even thickness, good appearance and has intact epithelium."



