As glaucoma specialists, we manage patients with all levels of disease, from ocular hypertensives on no medications to patients with advanced disease and limited vision. In many cases, medications aren’t sufficient to keep a patient’s intraocular pressure under control, so surgical intervention becomes necessary.
Fortunately, we have many more surgical options today than we did 10 or 20 years ago. That’s a good thing, in part because it allows us to take the severity of the patient’s disease into account when choosing a procedure. Each surgical option has different benefits and risks, so this is definitely not a case of “one size fits all.”
Unfortunately, many patients are still treated with less than ideal surgeries. We’ve seen patients in our clinic with mild, non-progressing glaucoma who had undergone a trabeculectomy. On the other hand, we’ve seen patients with advanced glaucoma who really did need a trabeculectomy but came to us having undergone a MIGS procedure.
The point is that it’s crucial to pick the right procedure for the right patient—and one of the most important determinants of the right procedure is the stage of the disease. (Note: The treatment algorithm described in this article is my own, based on my experience, so it’s only a guideline. Surgeons should use their experience and available technology to construct their own algorithm.)
Risk Matters
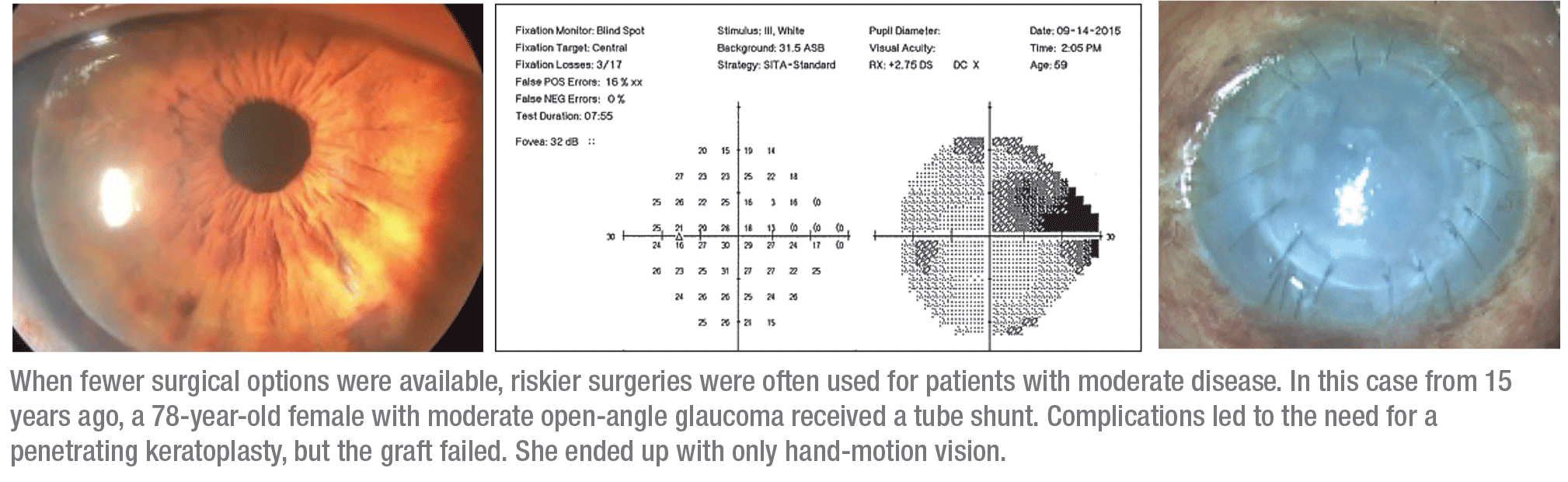
The dangers of using a procedure that’s more impactful than the patient needs are illustrated by a case we managed 15 years ago, when our surgical choices were far more limited. The patient was a 78-year-old female with primary open-angle glaucoma. Her pressure was 28 mmHg on three medications; her vision was 20/30, with a cup-to-disc ratio of 0.8. Her visual field revealed a superior arcuate scotoma and an inferior nasal step. (See illustration, facing page.) Her visual field damage was significant, but not advanced. She had what many doctors would consider moderate glaucoma.
At that time we felt that the best option for her was a tube shunt, so we implanted a Baerveldt Glaucoma Implant (Johnson & Johnson Vision, Santa Ana, California). As expected, her IOP was high during the first six weeks before the tube opened. Once it opened, the pressure de-creased significantly, to 4 mmHg. Un-
fortunately, at that point she developed a flat anterior chamber with a choroidal hemorrhage and effusion, requiring surgery to drain the choroid-al and re-form the anterior chamber.
She ended up with a pressure of 12 mmHg, which is very good; but because of the choroidal hemorrhage, effusion and flat chamber, she developed corneal failure and required a penetrating keratoplasty. Then, the graft failed and she declined further surgery. So she went from having 20/30 vision and a pressure of 28 mmHg to a pressure of 12 mmHg with only hand-motion vision. The aggressiveness of the surgery resulted in a poor outcome.
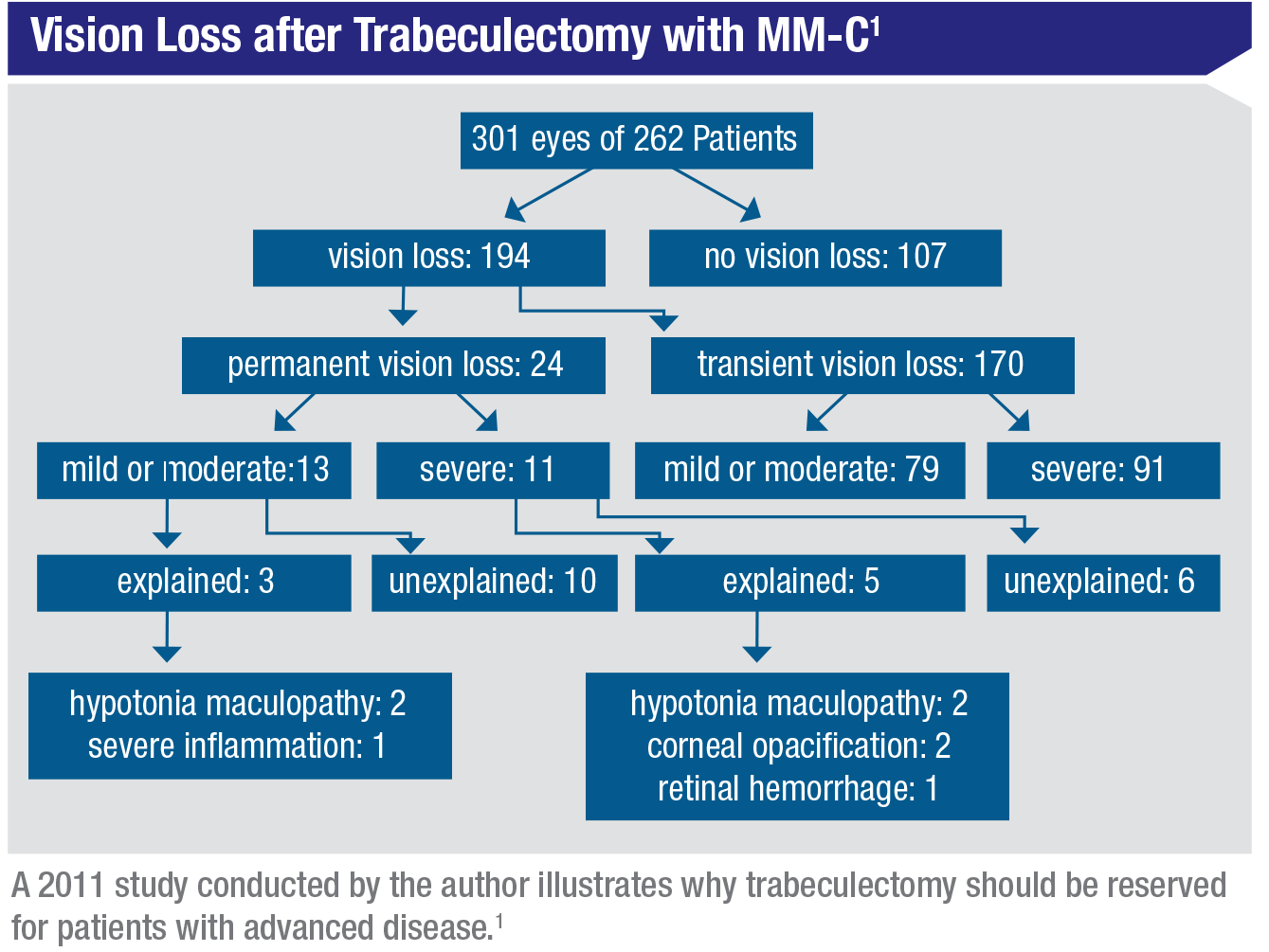
Surgeries such as tube shunts and trabeculectomies carry significant risks. In 2011 we published a study in which we looked at a large number of our own patients that had undergone trabeculectomy, to find out how they fared in terms of vision loss and recovery.1 Close to 65 percent of these patients experienced some vision loss during the postoperative period; fortunately, that loss was only permanent in 8 percent of them, so the majority recovered. (However, in some cases that recovery didn’t happen until one or two years after the surgery.) Meanwhile, severe, permanent vision loss occurred in about 4 percent of the patients; and of those, about half were unexplained. The causes we could explain included hypotony maculopathy, corneal opacification and retinal hemorrhage. The 2 percent that were unexplained had no complication or identifiable cause that we could find. (See table, p. 46.)
Along similar lines, a 2017 study examined the course of vision loss after Baerveldt aqueous tube shunt placement.2 The authors retrospectively reviewed 247 eyes of 222 patients who underwent Baerveldt implantations. Six months after surgery, 63 of the 247 eyes (25.5 percent) suffered long-term loss of three or more lines of Snellen visual acuity; in 24 of them, the visual loss was severe. In 18 cases, there was no identifiable cause of vision loss. Additionally, transient vision loss occurred in 76 of 242 eyes (30.8 percent). The authors concluded that a decrease in vision is not uncommon after Baerveldt surgery.
Today, of course, minimally invasive glaucoma surgeries, or MIGS, are an option. Still, we’re frequent-
ly asked why we don’t just do trabeculectomy in most patients; after all, the reasoning goes, it lowers pressure much more effectively than MIGS, and it’s considered the “gold standard.”
Yes, trabeculectomy is the gold standard when it comes to IOP lowering. But as these studies and the sample case demonstrate, there are serious risks associated with trabeculectomy and tube shunts, and those risks aren’t associated with MIGS. So, in our practice we now choose the surgery we’re going to perform based on a patient’s disease state—how severe the existing dam-age is, and the patient’s prognosis. That allows us to balance the patient’s need for pressure reduction with the risks of the surgery in question.
 |
Surgically Decreasing IOP
Currently, we have four different means to lower IOP surgically:
• Create transconjunctival filtration. We can do this via trabeculectomy or tube shunts, as well as newer options such as Xen, with the InnFocus/Santen Microshunt coming soon.
• Decrease aqueous production. These procedures alter the ciliary processes. The current alternatives include endoscopic cyclophotocoagulation; transscleral cyclophotocoagulation; and micropulse cyclophotocoagulation.
• Increase trabecular outflow. This can be done via an internal or external approach. The former group includes ab interno canaloplasty; excimer laser trabeculotomy; implanting a trabecular micro-bypass stent such as iStent or Hydrus; and trabeculotomy or goniotomy using an internal approach with a tool such as the Trabectome, Kahook Dual Blade or Goniotome.
• Increase suprachoroidal out-flow. Devices to accomplish this include the CyPass (Alcon, currently unavailable) and the iStent Supra (Glaukos) and MINIject (Staar) that are not cleared by the U.S. Food and Drug Administration. Given this list of options, the key issue becomes choosing the best alternative for the patient in front of you—in other words, individualizing the surgery for that particular patient, so that the procedure you perform isn’t more—or less—than what the patient needs.
To choose the ideal surgery, you need to weigh a list of factors. Probably the most important factor is target IOP. This should be based on the patient’s baseline IOP; the amount of glaucomatous damage already in evidence; and the rate at which the disease appears to be progressing. Beyond the target IOP, your choice should take into account:
• the anatomy of the eye;
• the health of the conjunctiva and sclera;
• the condition of the angle (including how closed or open the angle is, which will affect your ability to do angle-based surgery)
• whether the patient has had prior glaucoma surgeries;
• the risk of long-term infection;
• the risk of hypotony;
• the patient’s age and life expectancy;
• the patient’s ability to use glaucoma medications postoperatively; and
• the patient’s lifestyle and preferences.
The MIGS Myth
The key to getting the best out-comes is knowing when to do which type of glaucoma surgery. Solving that dilemma has become less straightforward with the advent of MIGS. Since MIGS is relatively new, we’re still trying to figure out how it fits in with the standard trabeculectomy and tube shunt options.
Many surgeons hesitate to resort to a MIGS procedure when a patient’s pressure is relatively high—for ex-ample, greater than 30 mmHg. Surgeons have heard that MIGS procedures generally produce a pressure drop of about 25 percent, so they calculate that they’ll only get a drop of 7 or 8 mmHg if the baseline pressure is 30 mmHg. Then, they may conclude that such a patient needs an aggressive form of surgery to achieve an acceptable pressure.
 |
Actually, this 25-percent drop with MIGS is a myth; regardless of the starting pressure, many MIGS procedures will leave your patient in the mid-teens. Thus, the higher the starting pressure, the more pressure drop you’re going to get. In fact, many MIGS procedures consistently produce pressures in the 15- to 16-mmHg range, and sometimes lower, regardless of the baseline pressure. For example, many patients starting with a pressure of 35 mmHg will end up at 16 mmHg following a MIGS procedure. That’s more than a 50-percent pressure reduction. So when deciding whether a MIGS procedure is appropriate, it’s not so much the amount of pressure lowering you should consider; it’s the target pressure you hope to achieve. This means that patients with very high baseline IOPs are often excellent candidates for MIGS, and a more aggressive form of surgery isn’t required.
Why isn’t this more widely known? For one thing, not too many patients start with pressure that high, so there’s not a lot of data about what happens following MIGS in these patients. However, we recently published a study demonstrating this pressure drop in 49 patients who began with a medicated IOP of 30 mmHg or higher.3 Twenty-eight of the patients were treated with Trabectome alone; 21 underwent Trabectome combined with phacoemulsification. Mean IOP was reduced from 35.6 ±6.3 mmHg to 16.8 ±3.8 mmHg at one year (p<0.01), and the number of medications dropped from 3.1 ±1.3 to 1.8 ±1.4 (p<0.01). (Nine patients required secondary glaucoma surgery and one patient had hypotony on day one, which resolved within a week.) So despite what you may have heard, MIGS procedures can be very effective in patients with high IOPs.
Of course, some patients need to reach a pressure lower than the mid-teens. In that situation, MIGS may not be appropriate, because while it can lower pressure more than many surgeons realize, it rarely will take it below the mid-teens. If you have a patient with advanced glaucoma with a pressure of 16 who is progressing, for example, that patient wouldn’t be a good MIGS candidate; that person needs a trabeculectomy to get down to a pressure of 10 mmHg or lower.
The point is that whether MIGS is appropriate depends less on the patient’s starting pressure, and more on whether you need to reach a very low target IOP with that patient. (The first patient we discussed, who had a pressure of 32 mmHg and a cup-to-disc ratio of 0.8, had moderate glaucoma. She would probably have done very well with Trabectome, Kahook Dual Blade or GATT, because she didn’t need to achieve a pressure of 10 or 11 mmHg.)
Individualizing Surgery
I think of glaucoma surgery options as a hierarchy. The first group of options would be those that use a laser to treat the trabecular meshwork (for example, SLT). The next group involves trabecular meshwork surgery, which can mean Schlemm’s canal dilation, trabecular stenting, trabecular removal by unroofing, or trabecular meshwork rupture with a procedure like GATT. The third group is suprachoroidal stents. (As everyone knows, this option is not available right now because of the recall of CyPass. However, I believe that CyPass will eventually be approved again for some treatments, and other suprachoroidal stents are in the pipeline.) The final group in my hierarchy is conjunctival filtration. The remaining type of surgery, aqueous suppression treatments, is a kind of wild card, because those procedures can be done at any disease stage. You can use them in early glaucoma combined with cataract surgery, as many surgeons do with ECP; and you can do them in advanced glaucoma patients who have failed multiple glaucoma surgeries.
Let’s consider these options in the context of patients at each level of disease severity:
• Ocular hypertension on no medications. For these patients I’d recommend doing SLT or cataract extraction by itself. (There’s good evidence from the OHTS study that cataract surgery in ocular hypertensives does help to lower IOP.)4
• Ocular hypertension on med-ical treatment. A patient in this situation would be thought of as a high-risk ocular hypertensive. If the patient has a cataract, you can still just remove the cataract, or you can do a minimally invasive procedure such as ab interno canaloplasty, or a trabecular stent. If the patient doesn’t have a cataract, you can still do SLT, an ab interno canaloplasty or a trabecular meshwork removal procedure such as Trabectome or Kahook Dual Blade.
• Mild open-angle glaucoma. If the patient has a cataract, you should consider trabecular removal, a trabecular bypass stent or performing ECP in addition to cataract surgery. If the patient doesn’t have a cataract, I’d suggest trabecular removal as a primary option, whether the patient is phakic or pseudophakic.
If a mild-glaucoma patient has al-ready had one of these procedures and it failed to achieve the goal, we can do Trab360, or GATT, which basically opens the trabecular meshwork 360 degrees. These patients might also benefit from a suprachoroidal stent, if they’re available. You can also consider adding an MPCPC or ECP procedure to suppress aqueous production.
• Moderate glaucoma. This group is more complicated, but can be treated with a wide range of options. If the patient has a cataract, your options are similar to the previous group: trabecular removal, a trabecular bypass stent, a suprachoroidal stent (if available), and also ECP combined with cataract surgery. If the patient doesn’t have a cataract, trabecular removal would be your primary choice, but you could also consider implanting a suprachoroidal stent (if available), and the Xen gel stent—using either the ab externo trans-conjunctival approach (XTC) or an internal approach. I find this way of implanting the Xen a bit easier than the internal approach, and there are two variations on it. For more moderate glaucoma, I recommend the ab externo transconjunctival approach. For more advanced glaucoma, I use the ab externo open conjunctival approach, which I call XEO.
If the moderate glaucoma patient has already failed one of these procedures, others can be performed as a secondary procedure.
• Severe open-angle glaucoma. The best option here depends a lot on the patient’s specific condition. If the patient has a cataract, you can do trabecular removal (or a supra-choroidal stent if available)—but only if the patient doesn’t need a pressure in the 10 to 12 mmHg range. You might also consider these options if the patient is at high risk for problems associated with filtration surgery.
If the patient doesn’t have a cataract, your options would include trabeculectomy, a tube shunt, and
implanting a Xen, ab externo, open conjunctival technique. If you pro-ceed with a tube shunt, my recommendation would be to use a non-valved shunt. These tend to achieve lower pressures, which we saw in the Ahmed vs. Baerveldt studies—although they’re also associated with a higher rate of complications.5,6
Incidentally, I’ve had many patients who clearly needed a trabeculectomy but specifically stated that they didn’t want one. They’d read about them online and were apprehensive about the risks. In those cases, if the patient needs a lower IOP, sometimes we combine MIGS procedures that work in different ways. If you combine an aqueous inflow procedure with a trabecular outflow procedure, for example, you may be able to get lower pressures than you would with just one of the MGIS procedures.
If a procedure has already failed, which option comes next depends in part on which one failed. Possible choices would include XEO or XTC; trabeculectomy; a tube shunt (I re-commend Baerveldt); a tube-shunt exchange (Baerveldt to Ahmed) if an Ahmed failed to achieve low-enough pressures; CPC or ECP; and a second tube (superior vs. inferior).
Ready for Anything
The bottom line is that your choice of surgery should be based on disease severity, and it should be patient-specific. Trying to use one surgical approach for every patient is a mistake. So, if you’re a glaucoma specialist, you should have several options in your armamentarium; that will allow you to appropriately manage the variety of patients and disease states you encounter.
I recommend that you be able to perform:
• a trabecular stent procedure, or an ab interno canaloplasty;
• a trabeculotomy or goniotomy procedure that can remove the tra-
becular meshwork—e.g., KDB, Tra-bectome, or possibly a trabeculotomy 360 or GATT procedure;
• an aqueous inflow procedure, whether it’s MPCPC, ECP or TSCPC;
• a conjunctival filtration device (right now that means Xen, with the InnFocus MicroShunt hopefully becoming an option soon);
• trabeculectomy and tube shunts. (For tube shunts, I recommend that you be familiar with both valved and non-valved options.)
Note that these recommendations are for glaucoma specialists. If you’re a general ophthalmologist, you don’t need to be able to offer all of these options, but you should be aware that they’re available. With that knowledge, if you realize that a particular procedure would be ideal for a patient, and it’s something you don’t offer, you can refer the patient for that procedure. REVIEW
Dr. Francis is a professor of oph-thalmology, as well as the Rupert and Gertrude Stieger Endowed Chair, at the Doheny and Stein Eye Institutes, David Geffen School of Medicine, University of California Los Angeles. He has consulted for Neomedix, Glaukos, BVI, NeOptix, Diopsys, Allergan and New World Medical; received grant support from Allergan, InnFocus, Santen, Alcon, Diopsys and Iridex; and has lectured for Bausch+Lomb and Aerie.
1. Francis BA, Hong B, Winarko J, et al. Vision loss and recovery after trabeculectomy: Risk and associated risk factors. Arch Ophthalmol 2011;129:8:1011-7.
2. Kim EL, Tran J, Töteberg-Harms M, et al. Vision loss and recovery after Baerveldt aqueous tube shunt implantation. J Ophthalmol 2017:4140305.
3. Akil H, Chopra V, Huang AS, Swamy R, Francis BA. Short-term clinical results of ab interno trabeculotomy using the Trabectome with or without cataract surgery for open-angle glaucoma patients of high intraocular pressure. J Ophthalmol 2017;2017:8248710.
4. Mansberger SL, Gordon MO, Jampel H, Bhorade A, Brandt JD, Wilson B, Kass MA; Ocular Hypertension Treatment Study Group. Reduction in intraocular pressure after cataract extraction: The Ocular Hypertension Treatment Study. Ophthalmology 2012;119:9:1826-31.
5. Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 2015;122:2:308-16.
6. Barton K, Feuer WJ, Budenz DL, Schiffman J, Costa VP, Godfrey DG, Buys YM; Ahmed Baerveldt Comparison Study Group. Three-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology 2014;121:8:1547-57.e1.



