As patients become increasingly particular and demanding about outcomes after cataract surgery, manufacturers have responded with a wealth of IOL options. As each comes through the FDA vetting process and receives approval, surgeons wonder what makes the lens different from the last. The latest to receive their approval are the IC-8 Apthera small-aperture IOL from AcuFocus and the SBL-3 Multifocal IOL from Lenstec, each with its own distinct design and ideal patient population. We spoke with some surgeons who were involved in the FDA study to find out how these lenses perform and what makes them stand out in a competitive marketplace.
AcuFocus IC-8 Apthera Small Aperture IOL
 |
|
AcuFocus IC-8 |
Cataract surgeons will now have a non-toric extended depth of focus IOL for patients with up to 1.5 D of astigmatism at their disposal. The IC-8 Apthera small aperture IOL from AcuFocus was recently FDA approved, and will be available this fall. Intended for implantation in the non-dominant eye, where the fellow eye has already undergone successful monofocal or monofocal toric IOL implantation (targeted for emmetropia),1 the IC-8 has been shown to mitigate the effects of presbyopia.
The IC-8 features a small central aperture, the FilterRing, to achieve an extended depth of focus. The lens is available in +10 D through +30 D in 0.5-D increments.1 The refractive target for the IC-8 should be -0.75 D.1
John Vukich, MD, founder and medical director of Summit Eye Care in Wisconsin, is a medical monitor for AcuFocus and has been involved with developing the prototype for this lens. He says the idea of using a pinhole to collimate light to extend depth of focus and eliminate blur is something all ophthalmologists learn as residents.
“This concept doesn’t require a great deal of explanation or leap of faith to understand why it works,” Dr. Vukich says. “As a practical matter, it’s an idea that’s been around forever.”
He says this technology gives surgeons a chance to meet the high expectations of cataract patients. “Cataract surgery is not just about removing a cloudy lens. There’s an expectation attached to it of having a predictable refractive outcome. Astigmatic correction still requires a toric lens, but now we have an option with a pinhole that will get up to 1.5 D or 1.75 D of correction of astigmatism,” says Dr. Vukich. “This has a large landing zone for emmetropia, and doesn’t have rotational or alignment issues.”
The FDA study revealed the IC-8 group was “statistically superior” in binocular UCIVA and UCNVA, as well as monocular DCIVA, compared to the group implanted with the control IOL.1 The visual performance of the IC-8 was demonstrated in a study of 105 patients that showed patients achieving UDVA of 20/23, UIVA of 20/24, and UNVA of 20/30 at six months.2
Visual symptoms were severe in a low percentage of test subjects, with more than 80 percent of subjects in both the IC-8 and control groups reporting they “never experienced symptoms,” “experienced symptoms but not bothered at all,” or were “a little bothered” by visual symptoms at 12 months postop.1
Dr. Vukich says patient satisfaction is high with this lens. “What you don’t get with the IC-8 are glare, ring halos, spider web halos, and all of the things that happen with the other multifocals and other methods of providing depth of focus,” he says. “Those lenses all have some optical aberrations that patients can notice. Many patients can disregard or ignore them, but there are certain patients who are never able to accept or adapt to the glare or halos with multifocality. So, if you can get rid of the aberrations that make patients unhappy or concerned, and with a lens that neutralizes up to 1.75 D of astigmatism, that’s a real win.”
Dr. Vukich believes this lens is a good option for any patient, and adds there is good evidence from international use that this lens works well for patients who have atypical corneas, corneal scars or are post-LASIK/post-PRK. “It’s not as easy to get a predictable outcome on those individuals, so I think this lens will provide a level of comfort with its big range. Even if we can get close postoperatively, the patient will still have good vision,” he says.
Adding to the IC-8’s strengths is its lack of learning curve, Dr. Vukich says. “Any surgeon who’s comfortable placing a one-piece acrylic lens in the capsular bag—and that should really be any cataract surgeon—will have no barrier to entry in terms of learning how to use this lens,” he opines.
The IC-8 is contraindicated for patients with a dilated pupil size less than 7 mm, in order to mitigate risks of secondary surgical interventions and IOL damage due to difficulty performing YAG laser treatments.1 Subjects with a history of retinal disease, high myopia, diabetes, macular disease, sickle cell disease, retinal tear and detachment, uveitis or retinal vein occlusion, or who are predisposed to retinal disease are not recommended for this lens.1
Although there’s been no shortage of IOLs coming to market, Dr. Vukich says it’s OK to have more than “one club in your bag,” so to speak. “There will be slight preferences that will tip the scale one way or the other, but having choice is better than not having choice,” he says. “We’re trying to get as close as we can to the natural function of a young human lens. We’re not there yet, but we’re getting closer, and this is a step in the right direction.”
Lenstec SBL-3 Multifocal IOL
Another newcomer to the IOL playing field is the SBL-3 (segmented bifocal lens) from Lenstec, whose segmented optic design will be the only one of its kind in the United States, according to the company.3 Its stated indications for use include improvement of near vision, while maintaining comparable distance and intermediate visual acuity, resulting in less reliance on spectacles.4
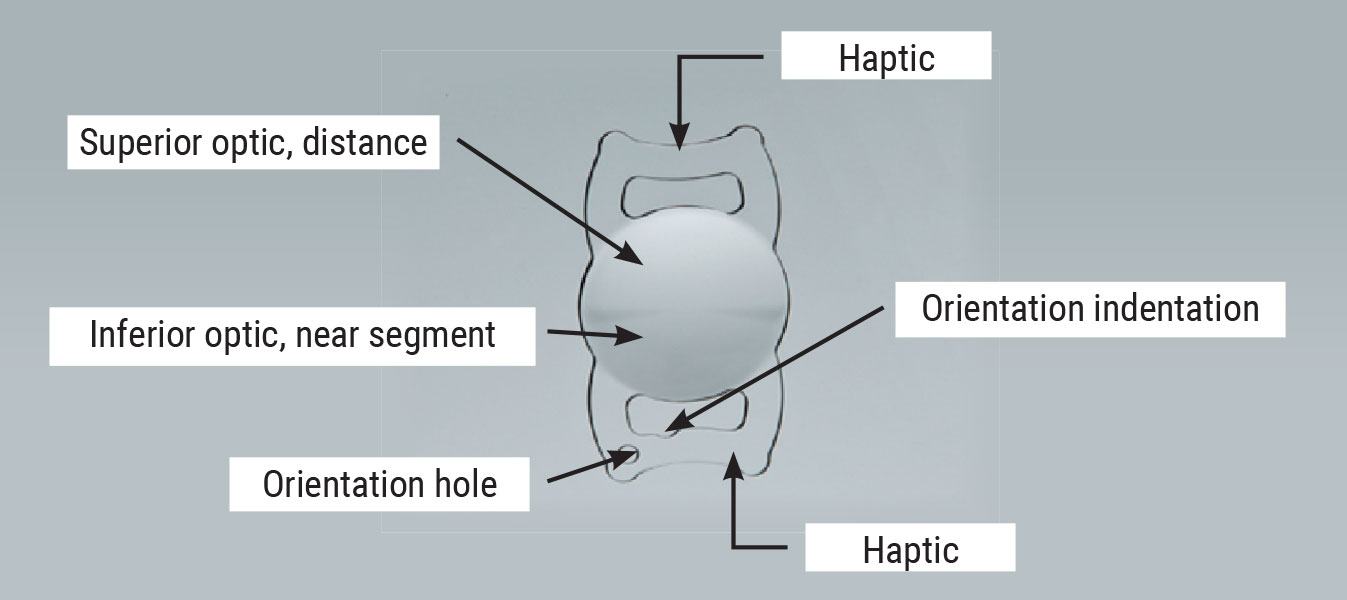 |
|
The Lenstec SBL-3 has a segmented optic with two power zones: The top is powered for distance and the bottom for near, much like bifocal glasses. |
Unlike other multifocal IOLs, the SBL-3 has a segmented optic with two power zones: The top is powered for distance, the bottom for near, much like bifocal glasses. There’s also a half-power ring around the near portion of the optic. The SBL-3 is available in 0.25-D increments with a power range of +15 to +25 D, and 0.50-D increments for +25 to +30 D.4
This lens has been available elsewhere, including the United Kingdom, Canada, South America and Germany. Many surgeons in the United States have been hearing from international colleagues about the SBL-3’s reported reduction of dysphotopsias, as well as how quickly patients adapt.
T. Hunter Newsom, MD, founder of Newsom Eye & Laser Center in Tampa, got his chance to work with the IOL as a part of the FDA study.
“We’d been hearing that patients were getting really good near and distance vision, and that the ability to see up close could occur very quickly, whereas with other multifocal technologies there is always that neuro-adaptation adjustment time,” Dr. Newsom says.
As part of the FDA study, some patients were implanted with a monofocal control IOL, but according to Dr. Newsom, patients could tell almost immediately which lens they’d received. “We’d do the surgery and practically 30 minutes postop patients would say, ‘I know I got the bifocal lens because I can read my watch right now,’” he recounts. “It wasn’t like I had to explain that they would adjust to the vision over the course of three months; it was more like putting on a pair of progressive glasses and very quickly starting to function very well.”
The FDA study revealed a “clinically meaningful difference” in the vision outcomes of the two lenses, with the SBL-3 representing a 20/25 mean visual acuity, versus 20/80 in the control group.4 When asked about using vision correction options (spectacles, contact lenses, increased font size on electronic devices, etc.), 93.3 percent of the SBL-3 group reported a reduced use, versus 25.5 percent in the control group. The SBL-3 group also had a much greater improvement in intermediate vision (93.9 percent) than the control group (45.3 percent).
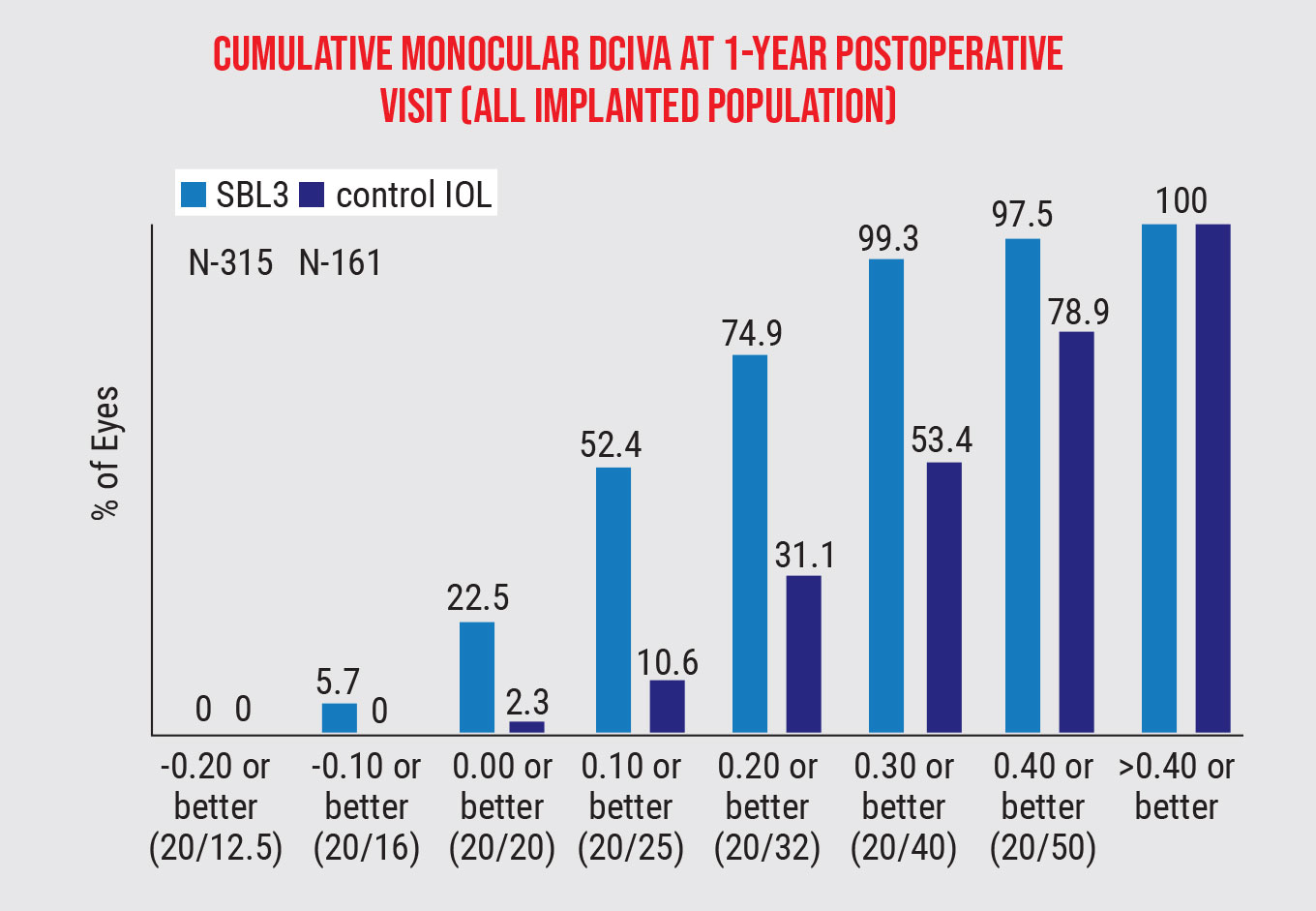 |
|
Figure 1. In the FDA study, the SBL-3 group showed an improvement in intermediate vision (93.9 percent) over the control (45.3 percent).4 |
Dr. Newsom says the SBL-3 aims to improve on some of the negative aspects of multifocal IOLs in general. “The problem with current multifocal technology is that you trade distance vision to gain at the near end, and you have a loss of nighttime quality vision,” he says. “The Vivity IOL, uses a novel approach that stretches the light, but it doesn’t give you as much near vision,” he says. “The SBL-3 uses the same technology as progressive spectacles. It’s not really special when you think about it that way, but it is special because you have to appreciate the engineering that went into creating a lens that’s one shape on top and another shape on the bottom, put together. All the other lenses use rings to make distance and near happen, and they have to do it circumferentially.”
Patients who are already wearing progressives would be natural fits for this lens, he continues, and he’s even willing to use the SBL-3 on patients who need good night-driving vision. “We put someone in the study who’s a professional long-haul truck driver and they’re still doing well at near and distance with no glasses,” he says. “I don’t think I would’ve ever put previous multifocal technology into someone like that,” Dr. Newsom says. “I think this lens is going to be more forgiving than our current multifocal technology and extend the patient-selection criteria.”
Dr. Newsom notes that there are pros and cons with the lens, as with anything else. “The lens doesn’t come in a toric, so if a patient has a large amount of astigmatism, you might have to dust off your LRI skills, which we’ve all done for years, but you need to be able to control the astigmatism with it,” he says. “And you may need to do adjustments or rotations to fine tune the refraction or results, just like we do with the current multifocal IOLs. You have to learn how this fits into your practice.”
Dr. Vukich is a consultant and medical monitor for AcuFocus. Dr. Newsom is a consultant for Lenstec.
1. IC-8 Apthera Intraocular Lens – P210005. FDA.gov. Accessed August 8, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf21/P210005B.pdf.
2. Dick HB, Piovella M, Vukich J, Vilupuru S, Lin L. Prospective multicenter trial of a small-aperture intraocular lens in cataract surgery. J Cataract Refract Surg 2017;43:7:956-968.
3. Lenstec SBL-3 multifocal intraocular lens for cataract surgery approved by FDA for sale in the U.S. Accessed July 29, 2022. https://www.lenstec.com/news/press-release-lenstec-sbl-3-multifocal-intraocular-lens-for-cataract-surgery-approved-by-fda-for-sale-in-the-us.
4. SBL-3 Multifocal Intraocular Lens – P200020. FDA.gov. Accessed August 8, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf20/P200020B.pdf.