Presentation
A 41-year-old man presented for decreased central vision in both eyes of approximately one week’s duration. He described sudden symptom onset, characterized as bilateral central scotomas that persisted with cross-covering. Review of systems was notable for upper respiratory symptoms starting two weeks ago and a positive COVID-19 PCR test one week prior to presentation. He denied any recent trauma or changes to medications. He reported normal vision at baseline in both eyes prior to the viral infection. He denied any additional sequelae of recent infection.
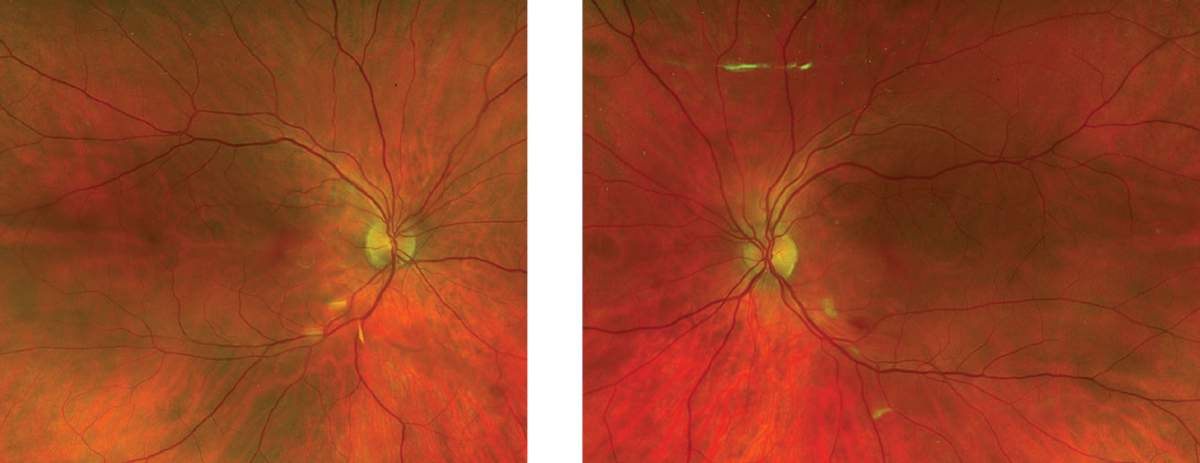 |
| Figure 1. Fundus photo of each eye without clear visible macular changes. A cotton wool spot and flame hemorrhage along the inferior arcade of the left eye are apparent. |
Medical History
Prior ocular history included cataract extraction with intraocular lens placement in both eyes. Otherwise, the patient denied any additional medical history or current medications. Prior surgeries included an appendectomy, cholecystectomy and hip surgery. Family history was notable for multiple relatives with development of cataracts at young ages.
Examination
The patient’s vital signs were stable and within normal limits. Visual acuity was 20/70 in the right eye and 20/40-2 in the left. Confrontational visual fields revealed a small central scotoma in both eyes. The patient didn’t demonstrate a relative afferent pupillary defect in either eye. Anterior slit lamp examination was unremarkable. On fundus examination, the only abnormalities seen were a cotton wool spot (CWS) and an adjacent flame hemorrhage along the inferior arcade of the left eye (Figure 1). Otherwise, posterior exam of each eye revealed clear vitreous and flat optic nerves with sharp borders and healthy rim tissue with cup-to-disc ratios of 0.3. The vessels were normal in caliber and contour, and the macula was flat without any evidence of exudates or pigmentary changes. There were no retinal detachments or breaks in the peripheral retina.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
Given the patient’s clinical exam findings, further diagnostic testing and imaging was performed. Initial hematologic work-up included: non-reactive HIV antigen/antibody; normal complete blood count and comprehensive metabolic panel; and normal lipid panel. Advanced diagnostic imaging included normal carotid and orbital doppler ultrasonography and unremarkable MRI/MRA of the orbit and neck. Near-infrared imaging revealed petaloid-shaped, well-demarcated darkening in the macula of both eyes (Figure 2). Optical coherence tomography of the macula of the right and left eye revealed outer retinal irregularities with disruption of the ellipsoid zone. A prominent hyper-reflective plaque extending from the outer plexiform layer to the outer nuclear layer was also noted in the left eye (Figure 2).
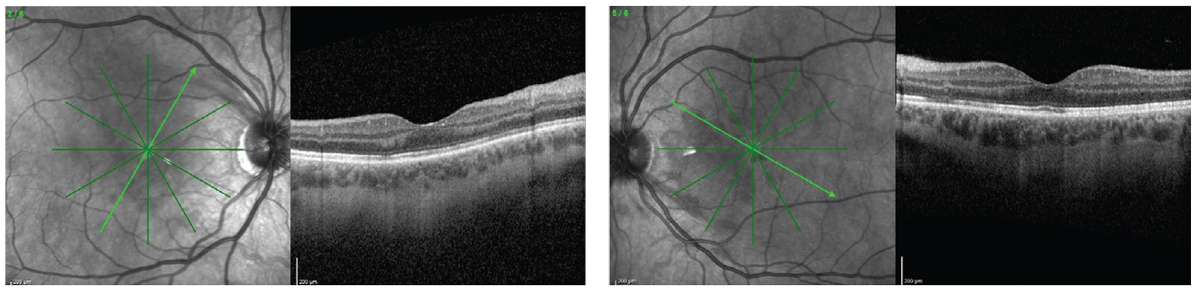 |
| Figure 2. Near-infrared reflectance of each eye demonstrates a dark, well-demarcated lesion in the macula. OCT shows outer retinal irregularities with ellipsoid zone disruption in each eye. A hyper-reflective band extending from the outer plexiform layer to the outer nuclear layer was identified in the left eye. |
The differential for this presentation was limited in scope, however it included acute macular neuroretinopathy (AMN), Krill’s disease, traumatic or whiplash retinopathy, and old retinal infarcts. However, the classic imaging patterns helped narrow down the diagnosis to acute macular neuroretinopathy.
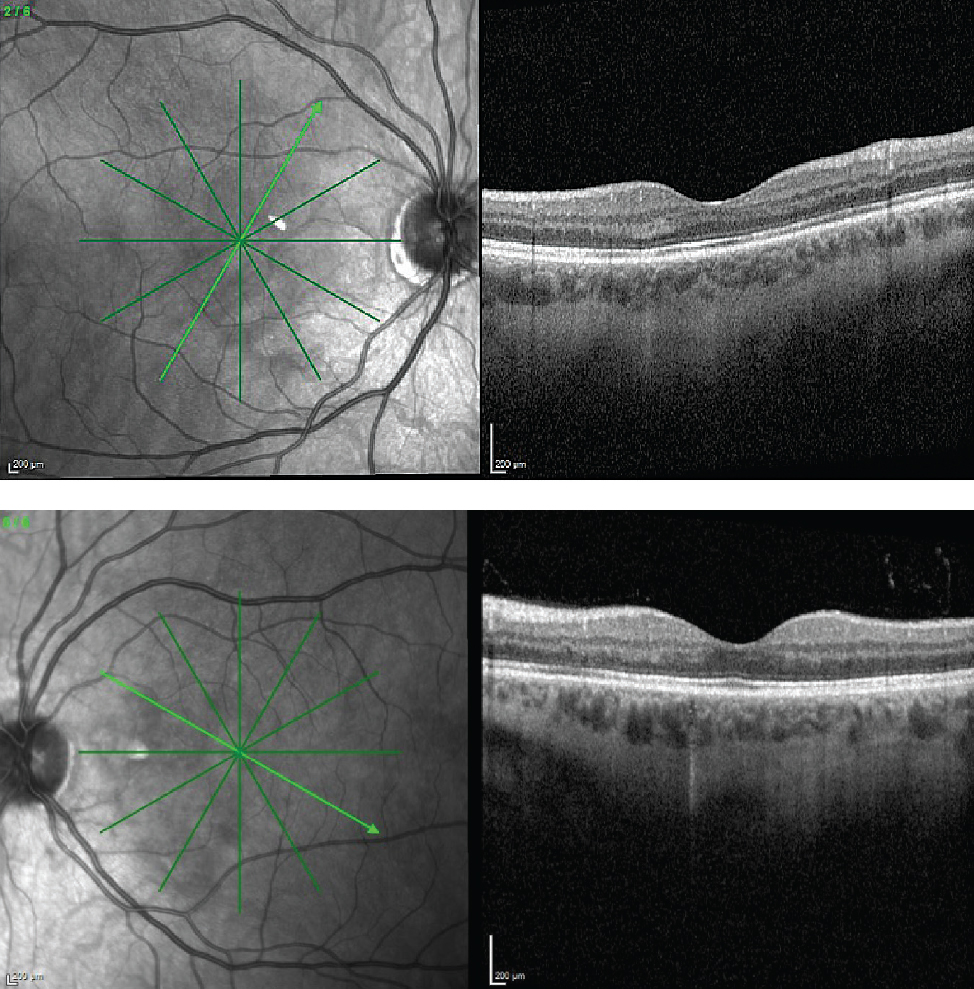 |
| Figure 3. Repeat OCT of both eyes at the six month follow-up visit reveals regression of outer retinal changes at previous areas of involvement. |
At this time, further serologic work-up including hypercoagulable investigation revealed a positive Factor V Leiden genetic analysis (heterozygous trait). Observation was advised as no treatment has been established for AMN. He was recommended to follow up with a primary care provider regarding his hypercoagulable work-up. On repeat examination at four weeks and six months, the patient was noted to have stable visual acuity and resolution of the previously noted CWS and hemorrhage in the fundus of the left eye. He continued to complain of a persistent paracentral scotoma in both eyes. Repeat OCT at six months revealed regression of the outer retinal changes of both eyes and improvement of the hyper-reflective plaque noted in the left eye (Figure 3). Per patient request for work clearance, 10-2 Humphrey visual fields were performed and revealed paracentral depressions in both eyes, though with limited reliability of the right eye (Figure 4).
Discussion
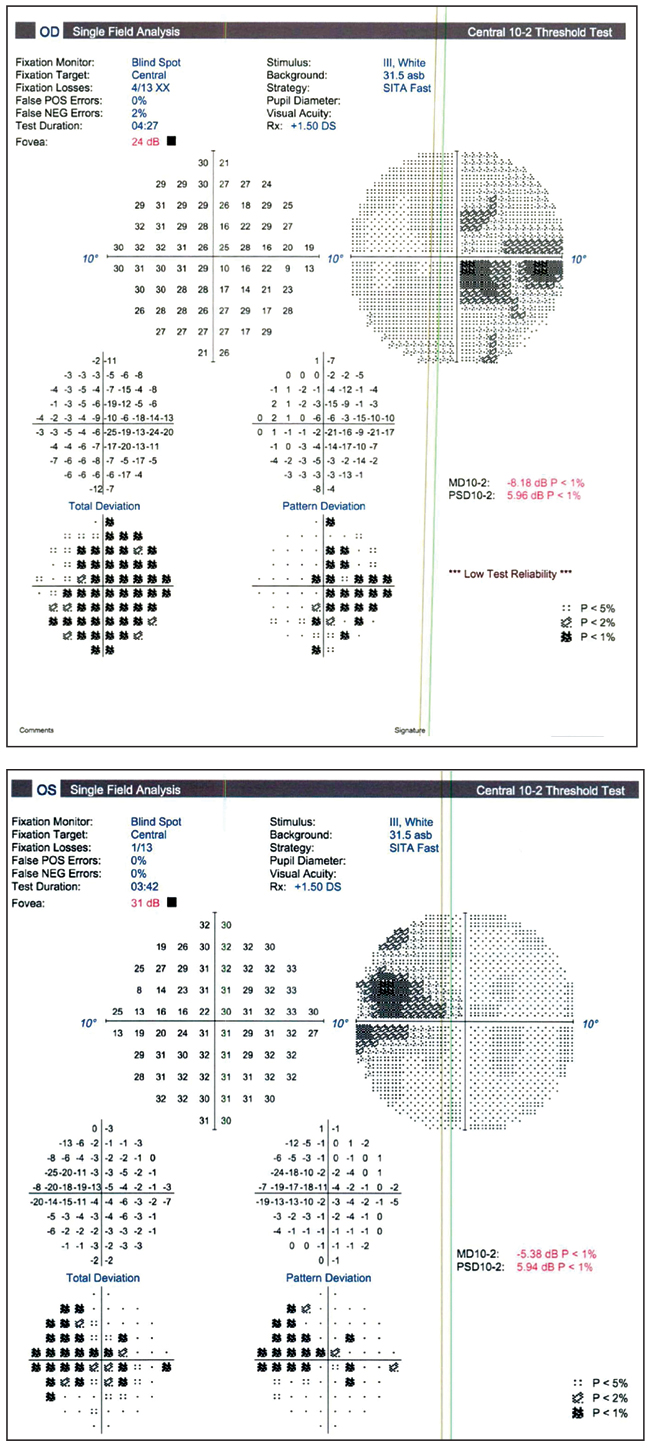 |
| Figure 4. 10-2 Humphrey visual field of each eye with paracentral changes. |
First described in 1975, AMN is believed to be a multifactorial disease, with a unifying vascular etiology, affecting the outer retina secondary to compromise of the deep capillary plexus resulting in visual field deficits.1-3 It generally affects younger patients and has been found to be more prevalent in females.2 Symptoms often follow different triggering events. The most commonly known trigger for AMN is a non-specific flu-like illness or fever with approximately half of patients reporting such symptoms prior to their disease.2 Other causes include the use of vasoconstrictors, hypotension/systemic shock, use of oral contraceptive pills and trauma.2 More recently, there have been several standalone case reports linking AMN with either acute infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 vaccinations. While the pathophysiology of such associations is yet to be delineated, it’s hypothesized to be precipitated by the hyperinflammatory and hypercoagulable state of COVID-19.4 This dysregulated state superimposed on the underlying thrombophilia from Factor V Leiden was presumed to be the trigger in our patient. To our knowledge, there are no previously published case reports of AMN in patients with a hereditary thrombophilia, and the pathophysiologic role of Factor V heterozygous trait remains unclear.
Most patients with AMN present with scotomas (estimated around 70 percent).2 The scotomas appeared to resolve in approximately half of the patients on long-term follow-up.2 Decreased vision is less commonly encountered, with more than 80 percent of patients presenting with visual acuity greater than 20/40 in the affected eye. Common findings on clinical exam include a wedge-shaped coloured macular lesion with the apex directed toward the macula.2 There are, however, patients who don’t demonstrate any appreciable changes on fundus exam and further imaging is required to identify the disease.2 More than half of patients with AMN present with bilateral disease.2
The most useful diagnostic tests in AMN are OCT and near-infrared reflectance. OCT findings in AMN include hyper-reflectivity of the outer retinal layers, ellipsoid zone changes, and outer nuclear layer thinning.2,5 These changes may be persistent on follow-up testing, with studies demonstrating persistent outer nuclear layer thinning in approximately 19 percent of affected eyes and ellipsoid zone disruption in approximately 9 percent.2
Near-infrared imaging, which is obtained in conjunction with OCT, is also an important diagnostic test that helps visualize subclinical disease. These images demonstrate a dark well-demarcated lesion proximal to the macula and often correspond to the areas of OCT changes and visual field defects.2 Fluorescein angiography has low utility in AMN with the majority of eyes (approximately 75 percent) demonstrating normal angiograms. However, hypofluorescence of the affected area has been described in approximately 20 percent of patients.2
Visual field testing can help delineate the scotoma and monitor for its improvement or resolution. These scotomas appear to follow the shape of the lesion visualized clinically, and persistent scotomas have been previously documented in nearly half of patients with AMN on long-term follow-up.2 Most reports of fundus autofluorescence in AMN show no abnormalities; however, hypofluorescence corresponding to the area of the lesion has been noted.2,6 Optical coherence tomography angiography has also been used in AMN. Findings include reduced deep capillary flow signal with evidence of flow recovery on follow-up.7 There is also evidence to suggest that the choriocapillaris may be involved in certain cases.8
In summary, AMN should be suspected in patients with central visual field deficits that have known risk factors. Although clinical examination can raise the suspicion for the disease, unique findings on multimodal imaging, including a well-demarcated darkening on near-infrared, outer retinal changes on OCT and central visual field defects on HVF, can help establish the diagnosis.
1. Bos PJ, Deutman AF. Acute macular neuroretinopathy. Am J Ophthalmol 1975;80:4:573-584.
2. Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, Cunningham ET. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol 2016;61:5:538-565.
3. Turbeville SD, Cowan LD, Gass JDM. Acute macular neuroretinopathy: A review of the literature. Surv Ophthalmol 2003;48:1:1-11.
4. Azar G, Bonnin S, Vasseur V, Faure C, Salviat F, Clermont CV, Titah C, Farès S, Boulanger E, Derrien S, Couturier A, Duvilliers A, Manassero A, Hage R, Tadayoni R, Behar-Cohen F, Mauget-Faÿsse M. Did the COVID-19 pandemic increase the incidence of acute macular neuroretinopathy? J Clin Med 2021;10:21:5038.
5. Fawzi AA, Pappuru RR, Sarraf D, Le PP, McCannel CA, Sobrin L, Goldstein DA, Honowitz S, Walsh AC, Sadda SR, Jampol LM, Eliott D. Acute macular neuroretinopathy: Long-term insights revealed by multimodal imaging. Retina Phila Pa 2012;32:8:1500-1513.
6. Yeh S, Hwang TS, Weleber RG, Watzke RC, Francis PJ. Acute macular outer retinopathy (AMOR): A reappraisal of acute macular neuroretinopathy using multimodality diagnostic testing. Arch Ophthalmol 2011;129:3:365-8.
7. Chu S, Nesper PL, Soetikno BT, Bakri SJ, Fawzi AA. Projection-resolved OCT angiography of microvascular changes in paracentral acute middle maculopathy and acute macular neuroretinopathy. Invest Ophthalmol Vis Sci 2018;59:7:2913-2922.
8. Thanos A, Faia LJ, Yonekawa Y, Randhawa S. Optical coherence tomographic angiography in acute macular neuroretinopathy. JAMA Ophthalmol 2016;134:11:1310-1314.



