Presentation
A 34-year-old Caucasian female presented for evaluation of blurry vision in both eyes for approximately one month in duration, with her left eye being more symptomatic than her right. In addition to her blurry vision, she also reported intermittent burning, irritation, and foreign body sensation in both eyes. There was no history of ocular trauma or contact lens wear. The review of systems was within normal limits. At the time of presentation, the patient was intermittently using artificial tears in both eyes, but without symptomatic improvement.
Medical History
 |
|
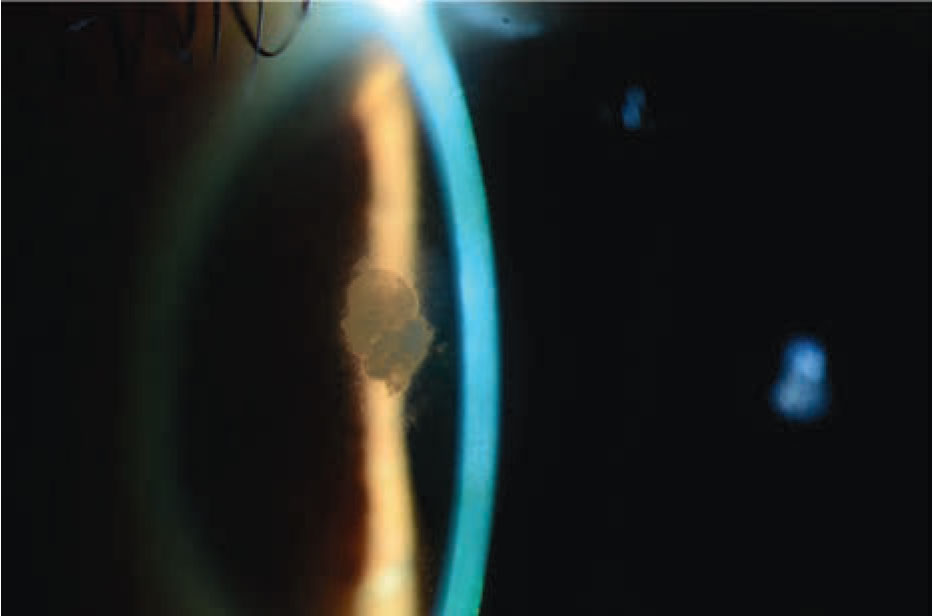
Figure 1. Slit lamp photograph demonstrating a superonasal, subepithelial infiltrate with surrounding haze. |
The patient’s past medical history was significant for dry-eye syndrome, diagnosed 10 years prior to presentation. Her past surgical history was significant for a bilateral small-incision lenticule extraction procedure performed in February 2019. Her family history was positive for Sjögren’s syndrome, lupus and diabetes mellitus on the maternal side. The patient was a non-smoker, consumed alcohol socially and denied illicit drug use. She denied allergies to medications. The patient’s medication list included topical tretinoin.
Exam
Visual acuity without correction was 20/20 in the right eye and 20/30+1, 20/25 pinhole in the left eye. Pupils were equal, round and reactive bilaterally with no relative afferent pupillary defect. Intraocular pressure was 12 mm Hg in both eyes. Visual fields were full to confrontation bilaterally. Extraocular motility was full bilaterally. Adnexa and eyelid examination was within normal limits bilaterally.
Slit lamp examination demonstrated diffuse 3+ punctate epithelial erosions (PEE) with decreased tear breakup time in both eyes. Additionally, the left eye demonstrated a superonasal paracentral opacity measuring 1.5 (h) x 1 (v) mm (Figure 1). Circumferential haze surrounding the opacity was noted. There were no associated infiltrates, edema or epithelial defects. The lesion appeared to be subepithelial and located at the SMILE interface. The remainder of the anterior segment and funduscopic examination in both eyes were unremarkable.
What is your diagnosis? What further workup would you pursue? The diagnosis appears below.
Work-up, Diagnosis, and Treatment
 |
The differential diagnosis for opacification within the SMILE interface includes diffuse lamellar keratitis, infectious keratitis and epithelial ingrowth. Given the length of the patient’s blurry vision, lack of pain, and history (albeit several years prior) of a SMILE procedure, epithelial ingrowth was highest on the differential with diffuse lamellar and infectious keratitis being less likely.
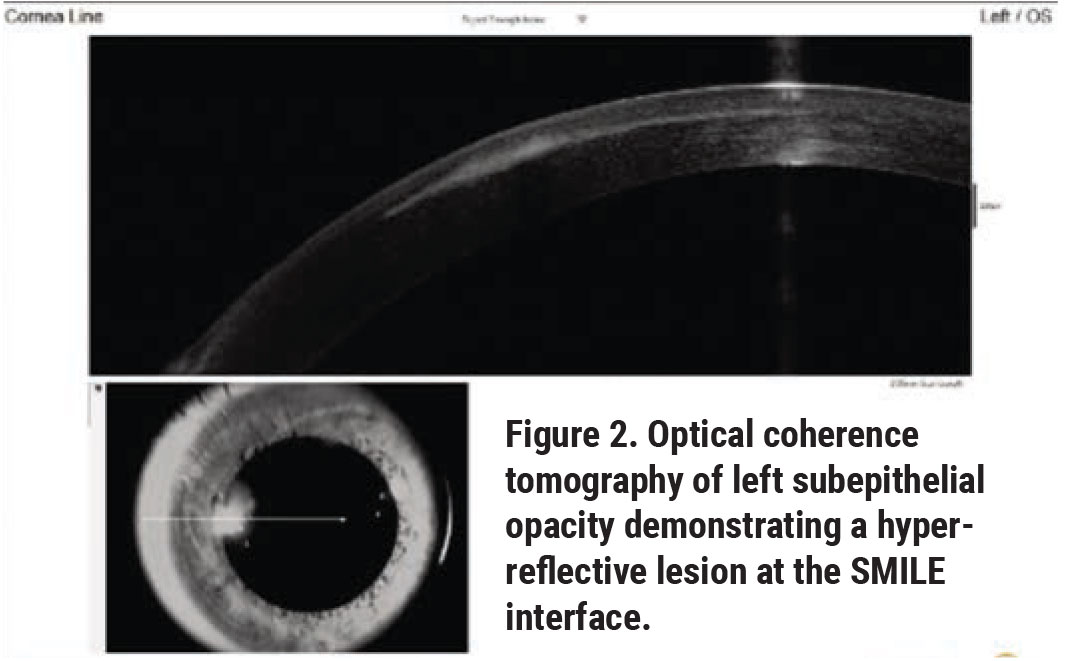
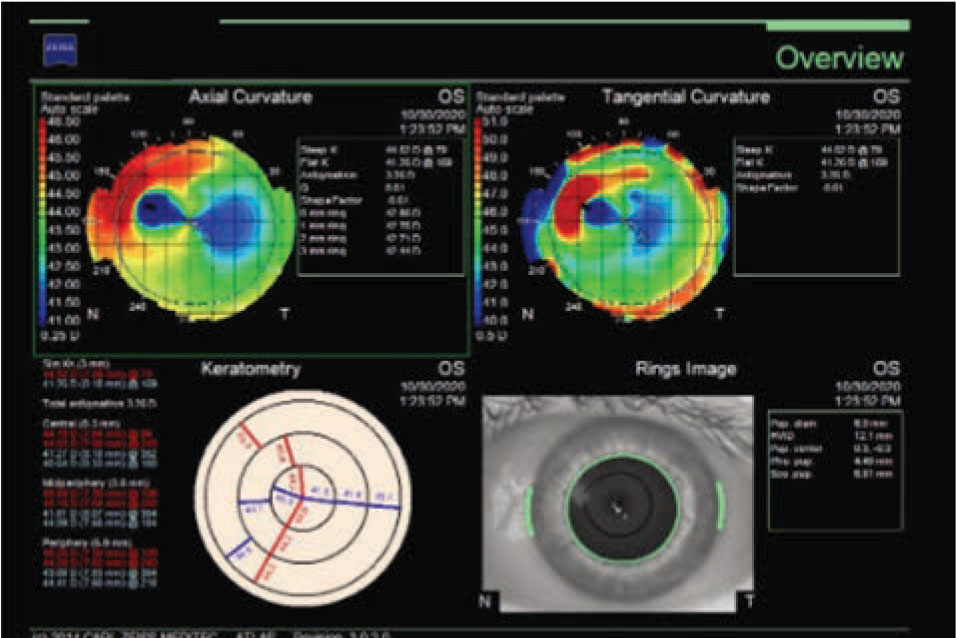
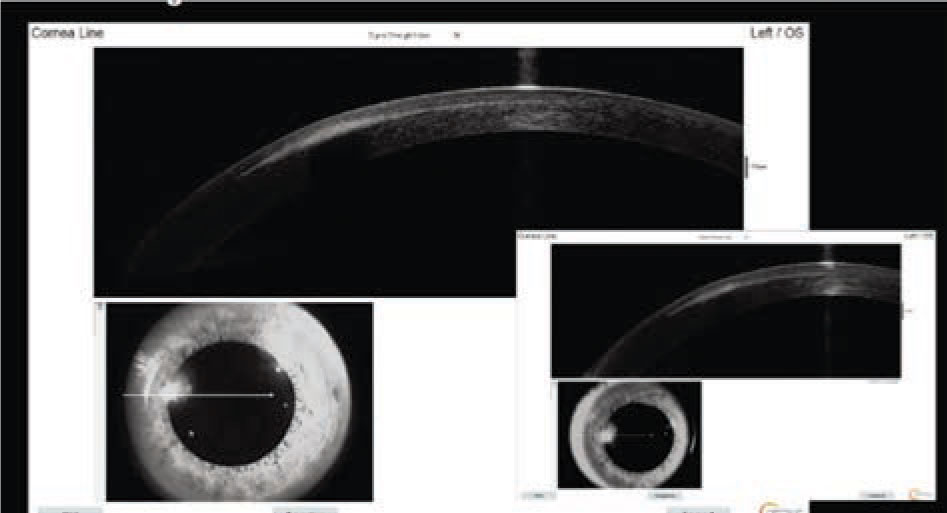
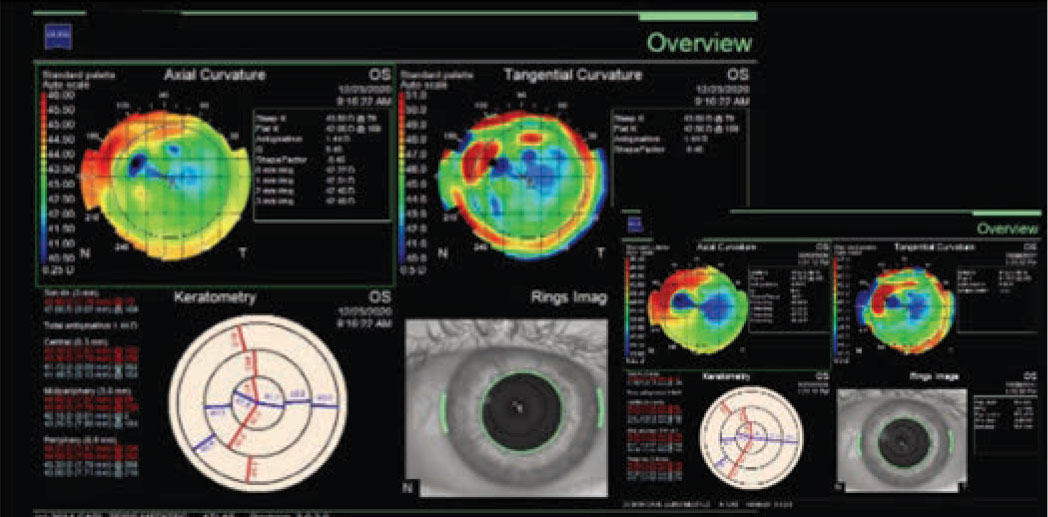
Optical coherence tomography of the left eye demonstrated a hyper-reflective lesion at the SMILE interface (Figure 2). Corneal topography was subsequently obtained, which documented flattening at the location of the opacity with surrounding elevation (Figure 3); the left eye was also noted to have increased astigmatism compared to the right eye.
 |
|
Figure 3. Corneal topography of left eye demonstrating corneal flattening at the site of the subepithelial opacity with surrounding corneal elevation. |
Given the constellation of slit lamp, OCT, and corneal topography findings it was determined that the corneal opacity was likely secondary to epithelial ingrowth following the patient’s SMILE procedure. Treatment options were discussed with the patient, including observation, surgical intervention, and laser treatment. Given the chronicity and relative mildness of the patient’s symptoms, she was given the option to think about her treatment options and follow-up in two months.
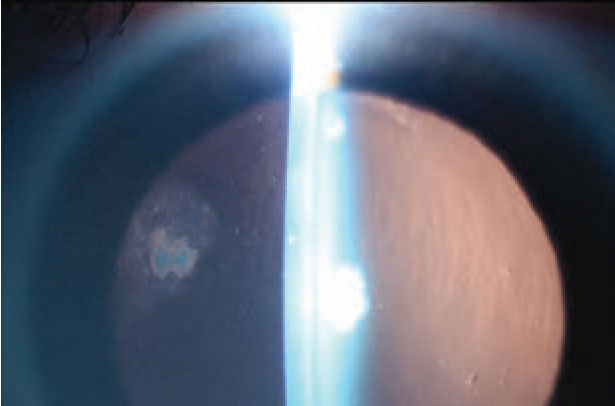
The patient followed up two months later and continued to complain of persistent dryness and irritation in both eyes. She also reported blurry vision which was worse in her left eye than her right, though she noted that it had slightly improved. Visual acuity without correction was 20/25 in both eyes. Slit lamp exam was notable for 3+ PEE and decreased tear breakup time in both eyes; once again, a subepithelial opacity at the SMILE interface was noted in the left eye. On close observation, a reduction in both the size of the opacity and the surrounding haze was noted compared to the initial visit (Figure 4).
 |
|
Figure 4. Slit lamp photograph |
Repeat OCT demonstrated a hyper-reflective lesion at the SMILE interface of the left eye which had slightly decreased in size compared to the initial OCT (Figure 5). Corneal topography was repeated, showing less flattening at the site of the opacity (Figure 6). Astigmatism was still greater in the left eye, however the difference between the left and right eyes had decreased from the initial visit.
With all three exam modalities showing recession of the presumed epithelial ingrowth, it was decided to further observe the opacity and see if it continued to self-resolve with a plan for surgical removal if no significant reduction was noted. The patient was agreeable and consented for surgery with a tentative date shortly after a scheduled two-month follow up.
Discussion
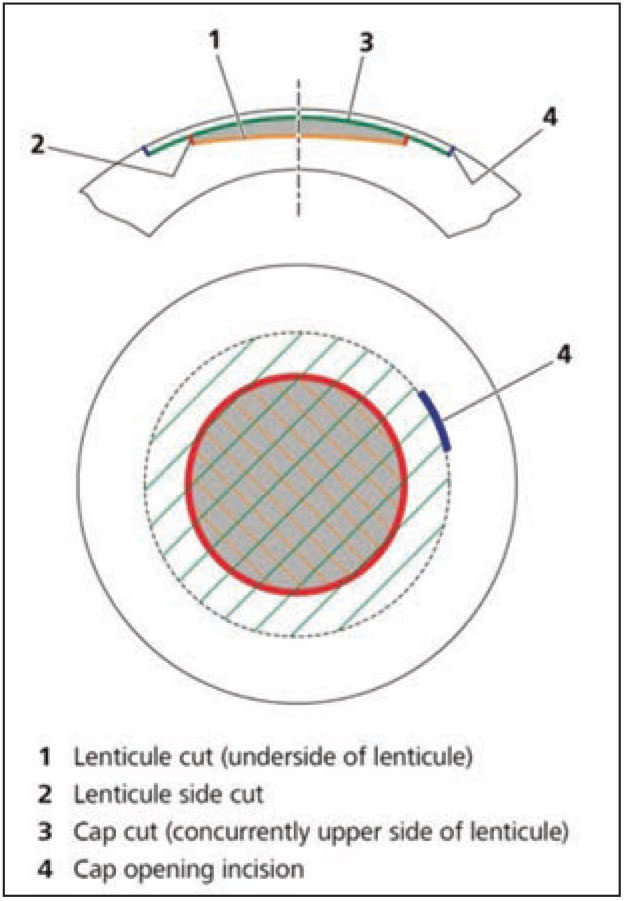
First introduced in 2008 and gaining FDA approval for myopic correction in 2016, the small-incision lenticule extraction procedure is a recently developed refractive surgery procedure that’s an alternative to LASIK and PRK. The SMILE approach focuses on the creation and removal of a refractive lenticule without epithelial removal or flap creation. The intrastromal lenticule is created with a femtosecond laser, and is composed of an upper (known as the cap) and lower interface. A 2 to 3 mm side incision, for removal of the lenticule, is also made.1 The side incision also serves to connect the cap to the corneal surface (Figure 7). Currently, in the United States SMILE is approved for correction of up to 10 D of myopia and 3 D of astigmatism.
Multiple studies have demonstrated the efficacy of SMILE. The prospective multicenter study which led to FDA approval studied 357 eyes treated with SMILE and followed patients for up to 12 months. The study found 95.3 percent (n=289) of treated patients to be within 0.5 D of emmetropia; 89 percent (n=270) of patients had UDVA of 20/20 or better at the 12-month follow-up.3
|
| Figure 5. Optical coherence tomography of the left subepithelial opacity demonstrating reduction in size of the hyper-reflective lesion at the SMILE interface (inset of OCT from initial visit). |
A study performed at the Singapore National Eye Institute compared refractive predictability in patients who underwent SMILE in one eye and LASIK in the fellow eye. At the three-month follow-up, 99 percent of SMILE eyes and 97 percent of LASIK eyes were within 1 D of emmetropia; these results stayed statistically unchanged at the 12-month follow-up, with 99 percent of SMILE and 99 percent of LASIK eyes being within 1 D of emmetropia. Additionally, this study also found that both SMILE and LASIK achieved an uncorrected distance visual acuity of 20/20 or better at a similar rate after a year (SMILE: 84 percent; LASIK: 87 percent).4
It’s been surmised that because SMILE doesn’t violate the anterior stroma in the manner that LASIK and PRK do, it offers patients enhanced postop corneal biomechanical properties. A non-linear regression analysis using depth-dependent tensile corneal strength found that postoperative tensile strength would be greatest in SMILE compared to PRK and LASIK, although a formal study has yet to use this regression to prove superior postop tensile strength.2,5 SMILE’s minimal violation of the anterior cornea has led to the hypothesis that it provides faster postop corneal nerve innervation allowing for enhanced corneal sensation and reduction in ocular surface disease. A study comparing eyes treated with SMILE and LASIK found that at one week, one month, three months and six months postop central corneal sensation was decreased from baseline in both groups, however the reduction was greater in eyes treated with LASIK.6 Using confocal microscopy, a study demonstrated that LASIK produced a greater decrease in corneal nerves compared to SMILE;7 an additional study describes increased subbasal nerve fiber density in eyes treated with SMILE vs LASIK at three months postop.8 A report comparing dry-eye parameters between SMILE and LASIK found that the Schirmer’s test and tear breakup time were greater in SMILE patients than LASIK patients.9 (It should be noted that other studies comparing dry-eye parameters found no statistically significant difference between the two procedures.)
|
| Figure 6. Corneal topography of the left eye demonstrating reduction of corneal flattening and surrounding corneal elevation at the site of the subepithelial opacity (inset of corneal topography from initial visit). |
In addition to its efficacy and enhanced biomechanical/corneal innervation properties, SMILE has also been proven to be safe. In the FDA clinical trial, 2.2 percent of eyes (n=8) experienced postoperative adverse events, none of which negatively impacted the patients’ long-term visual acuity.3 A clinical control cohort study at the Department of Ophthalmology at Aarhus University in Aarhus, Denmark, followed 1,800 post-SMILE eyes and found 1.5 percent of them (n=24) had reduced best-corrected distance visual acuity from baseline at the three-month follow-up visit. By 12 months, however, all 24 eyes were within one line of their preop BCDVA. The study also found that 9.78 percent of eyes (n=154) experienced postoperative complications, with the most common adverse events being trace haze and corneal surface dryness. In both the FDA clinical trial and Danish study, epithelial ingrowth was found to be a rare complication. The FDA and Danish studies found that 0.6 percent (n=2) and 0.64 percent (n=10) of eyes experienced epithelial ingrowth, respectively. Of the 12 eyes found to have ingrowth between the two studies, 11 had spontaneous resolution with no effect on BCDVA. One eye required surgical intervention at the 12-month follow-up, with a resultant UDVA of 20/20.10
One potential drawback with SMILE is the case in which a patient needs an enhancement. SMILE enhancements aren’t FDA-approved, and surgeons usually have to either cut a LASIK flap or perform surface ablation to enhance a SMILE patient, potentially negating some of SMILE’s purported benefits compared to these other procedures in the process.11
The exact cause for epithelial ingrowth following SMILE procedure isn’t known, however there are two prevailing hypotheses. The first is that when making the small side incision for the lenticule extraction a tract is formed which allows corneal epithelial cells to migrate and form a nest at the surgical interface. The other hypothesis states that, during the procedure, epithelial cells are spread and ultimately planted at the interface during lenticule extraction.
Given the rarity of epithelial ingrowth following SMILE, no studies exist providing the appropriate approach to handling the complication. There are few case reports that describe both progressively increasing and visually significant epithelial ingrowth, and they describe varying approaches to correct it.
|
| Figure 7. Schematic demonstrating the geometry of the SMILE procedure. |
One case study described two patients who presented with subjective blurry vision following SMILE, with the slit lamp exam demonstrating a corneal opacity at the SMILE interface site in both patients. Subsequent OCT imaging found both patients to have an incisional tear and nest of epithelial ingrowth at the interface. Given the combination of subjective blurry vision and presence of ingrowth, it was decided to pursue surgical removal of the epithelial cells. The procedure consisted of using basic saline solution to separate the epithelial cells from the interface, followed by scraping of the cells with a blunt spatula, ensuring their complete removal. Once the cells were separated from the interface, 27-ga. vitreoretinal forceps were used to completely remove them. The interface was subsequently closed with 10-0 nylon sutures and a bandage contact lens was placed for a week. Both patients were closely followed, and at the one-month follow-up both were found to have BCVA 20/16 with complete stability of their SMILE interface.12
Another case report describes a 40-year-old female who presented with progressive increase in the size of epithelial ingrowth at the SMILE interface on OCT over the span of seven months. Though the patient’s BCVA remained 20/20, it was determined that the epithelial ingrowth would be treated given its progressive enlargement. The providers describe using a Nd:YAG laser to break up the collection of epithelial cells and eliminate the epithelial collection at the three-month follow-up visit. The patient continued to have complete resolution of epithelial ingrowth with consistent BCVA 20/20 in all subsequent follow-ups.13
In summary, the SMILE procedure is a minimally invasive refractive surgery technique with an efficacy and safety profile similar to the more established procedures LASIK and PRK. Studies have shown that SMILE may offer patients postoperative benefits compared to other refractive surgery techniques, including increased corneal tensile strength, improved corneal sensation/innervation and a potential reduction in postop ocular surface disease. Epithelial ingrowth is an extremely rare post-SMILE complication that often self-resolves with no long-term effect on vision. In the rare cases requiring intervention, both surgical and laser therapies have been proven to remove the nest of epithelial cells, maintain interface stability and ensure no decompensation of patient vision.
1. Liu Y-C, Pujara T, Mehta JS. New Instruments for lenticule extraction in small incision lenticule extraction (SMILE). PLoS ONE 2014;9:12:e113774. doi:10.1371/journal.pone.0113774
2. Reinstein DZ, Archer TJ, Gobbe M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye and Vis 2014;1:3.
3. Dishler JG, Slade S, Seifert S, Schallhorn SC. Small-incision lenticule extraction (SMILE) for the correction of myopia with astigmatism: Outcomes of the United States Food and Drug Administration premarket approval clinical trial. Ophthalmology 2020;127:8:1020-1034.
4. Ang M, Farook M, Htoon HM, Mehta JS. Randomized clinical trial comparing femtosecond LASIK and small-incision lenticule extraction. Ophthalmology 2020;127:6:724-730.
5. Randleman JB, Dawson DG, Grossniklaus HE, McCarey BE, Edelhauser HF. Depth-dependent cohesive tensile strength in human donor corneas: Implications for refractive surgery. J Refract Surg 2008;24:S85–S89
6. Demirok A, Ozgurhan EB, Agca A, Kara N, Bozkurt E, Cankaya KI, Yilmaz OF. Corneal sensation after corneal refractive surgery with small incision lenticule extraction. Optom Vis Sci 2013;90:1040–1047.
7. Vestergaard AH, Gronbech KT, Grauslund J, Ivarsen AR, Hjortdal JO. Subbasal nerve morphology, corneal sensation, and tear film evaluation after refractive femtosecond laser lenticule extraction. Graefes Arch Clin Exp Ophthalmol 2013;251:2591–2600.
8. Li M, Niu L, Qin B, Zhou Z, Ni K, Le Q, Xiang J, Wei A, Ma W, Zhou X. Confocal comparison of corneal reinnervation after small incision lenticule extraction (SMILE) and femtosecond laser in situ keratomileusis (FS-LASIK) PLoS One 2013;8:e81435. doi: 10.1371/journal.pone.0081435.
9. Xu Y, Yang Y. Dry eye after small incision lenticule extraction and LASIK for myopia. J Refract Surg 2014;30:186–190.
10. Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology 2014;121:4:822-8.
11. Siedlecki J, Luft N, Dirisamer M, et al. Enhancement options after myopic small-incision lenticule extraction (SMILE): A review. Asia Pac J Ophthalmol (Phila) 2019;8:5:406.
12. Kamiya K, Takahashi M, Shoji N, Naruse S. Two cases of epithelial ingrowth after small incision lenticule extraction. Am J Ophthalmol Case Rep 2020 9;19:100819.
13. Piccinini P, Vida R, Piccinini R, et al. Epithelial implantation treatment after small-incision lenticule extraction. J Cat Refract Surg 2020;46:4:636-640.



