Intravitreal anti-vascular endothelial growth factor therapies have ushered in a new era in the treatment of neovascular age-related macular degeneration. Currently, the use of intravitreal bevacizumab (Avastin) and ranibizumab (Lucentis) has transformed the AMD landscape, allowing ophthalmologists to prevent significant vision loss in the majority of patients who develop the disease and even salvage vision in a subset of those.1,2 However, these medications have transient effects, primarily controlling exudation temporarily without leading to complete involution of the choroidal neovascular membrane (CNVM). Clinical trials with Lucentis clearly showed that monthly injections were superior to quarterly dosing.3 Therefore, repeated injections for an indefinite period of time currently appear to be the rule in managing these patients. As a result, an emerging area of interest is the use of combination therapies to provide a more sustained treatment benefit. The potential synergy between the anti-VEGF agents and localized ionizing radiation is currently being explored. The rationale and clinical findings will be the focus of this article.
Historical Perspective
Radiation as a treatment modality for neovascular AMD is not a new concept. Local radiation therapies have been shown to prevent proliferation of vascular tissue via inhibition of neovascularization.4,5 Following exposure to low-dose radiation, vascular endothelium undergoes morphologic and DNA changes, inhibition of replication, increased cell permeability, and ultimately apoptosis.6-13 In addition, the proliferation of fibroblasts and formation of fibrosis, often seen in end-stage neovascular AMD, is inhibited.
Proliferating endothelial cells, as found in CNV, are preferentially more sensitive to radiation treatment compared to the nonproliferating capillary and larger-vessel endothelial cells.14 In fact, several clinical studies have supported the use of external beam ionizing radiation alone for the treatment of neovascular AMD. The Age-Related Macular Degeneration Radiotherapy Trial suggested that external beam radiation with five fractions of 4 Gy had a modest but short-lived benefit in preserving visual acuity.15 Another randomized clinical trial enrolled 161 patients with subfoveal CNV. Patients randomized to four fractions of 2 Gy (8 Gy total) and four fractions of 4 Gy (16 Gy total) showed a reduction in visual acuity loss during the 18 months of follow-up compared to control.16 However, others have shown no benefit as monotherapy.17 One randomized clinical trial of 83 eyes with subfoveal CNV showed no benefit or harm of seven fractions of 2 Gy (14 Gy total) by one year follow-up.18 Another trial involving 203 patients randomly assigned to six fractions of 2 Gy (12 Gy total) versus observation showed no significant benefit in terms of moderate or severe vision loss at 24 months.19 A review of 11 randomized controlled clinical trials involving 1,078 patients with external beam dosages ranging from 7.5 to 24 Gy revealed that most trials found effects that favored treatment, though the re-sults were not always significant.20 The authors concluded that more evidence was needed to support external beam radiotherapy as an effective treatment for neovascular AMD.
Epimacular Brachytherapy
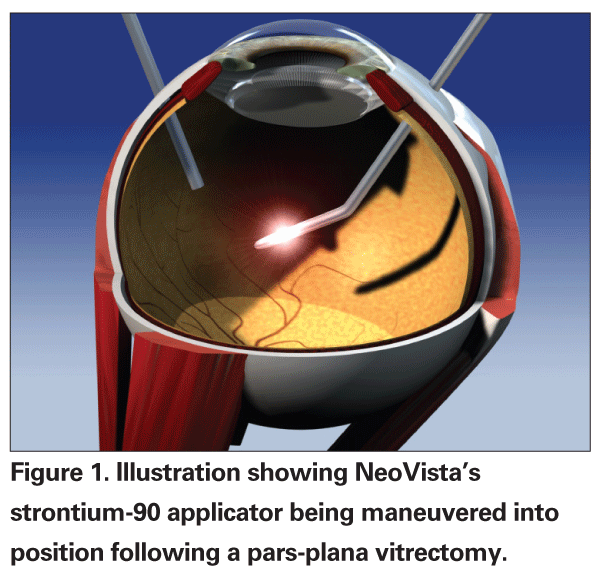
Besides external beam radiotherapy, a novel intraocular strontium-90 applicator (NeoVista,
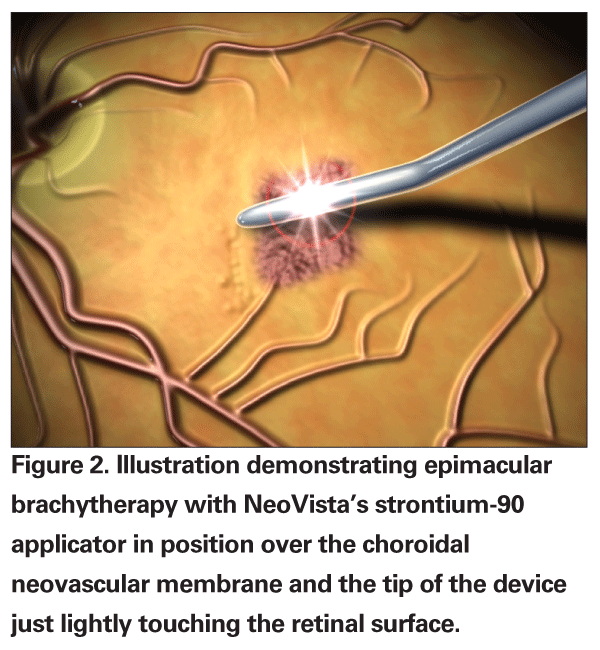
A nonrandomized, multicenter feasibility study with this device involved treatment-naïve patients with subfoveal CNV due to AMD.21 Patients received a single treatment of 15 Gy or 24 Gy using the strontium-90 applicator. At the 12-month follow-up visit, all patients had lost less than three lines of vision. Two of the four patients (50 percent) in the 15-Gy group and 13 of the 17 patients (76 percent) in the 24-Gy group improved or maintained their visual acuity. Five of 17 patients (29 percent) in the 24-Gy group gained three or more lines in visual acuity. Overall, the 24-Gy group had a mean gain in visual acuity of +10.3 letters at 12 months.
Potential Benefits of PPV for AMD
Epimacular brachytherapy requires the completion of a pars plana vitrectomy in order to safely insert the strontium-90 applicator directly over the macula. This minimizes radiation exposure to many other ocular structures. Vitrectomy alone has also been reported to have a potential effect on CNV regression in some patients.22 Many feel that this is related to a change in oxygen distribution in the eye with potentially increased oxygen tension to the retina.23 Moreover, radiation is effective through the formation of free radicals that can then induce irreparable damage at a cellular level. Oxygenation may therefore enhance the therapeutic effect of the radiation, similar to the use of tissue oxygenation as an enhancer of radiation in the treatment of certain cancers.24,25
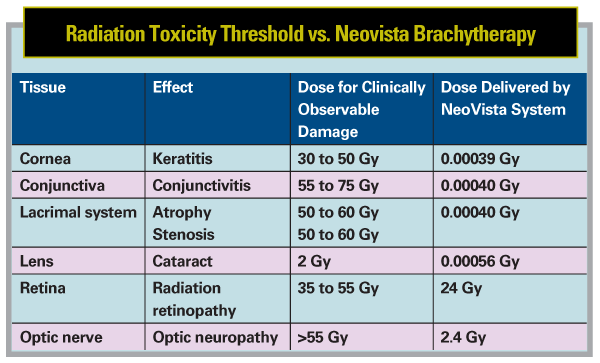
Combination Therapy
Even the clinical studies with ionizing radiation alone that have shown a benefit still fall short of the results found in the Lucentis trials. The problem with intravitreal anti-VEGF therapies alone is that they do not seem to cause permanent regression of the CNV with recurrence often developing once the injections are stopped. Radiation therapy holds the potential promise of more permanent CNV closure. Combining the two treatments may have a synergistic effect, allowing long- term visual benefits with fewer required intravitreal injections.
The first clinical study which demonstrated this potential benefit again involved the intraocular strontium-90 applicator. This device delivered a targeted dose of 24 Gy to the center of the CNV using the epimacular brachytherapy technique described before. A prospective, non-randomized, multicenter study was performed on 34 treatment-naïve patients with subfoveal CNV due to AMD using this device in combination with intravitreal Avastin.26 The 12-month results showed that 91 percent of patients lost <three lines of vision and 38 percent gained ≥three lines of vision. No further injections beyond two initial ones given one month apart were required in 26 of 34 patients (76 percent). A Phase-III multicenter, randomized, controlled study known as CABERNET (CNV Secondary to AMD Treated with Beta Radiation Epiretinal Therapy) is currently underway to evaluate the effects of this strontium-90 applicator when combined with two intravitreal Lucentis injections (immediately after the epimacular brachytherapy and one month postop) compared to monthly Lucentis monotherapy in treatment- naïve patients with subfoveal CNV. Enrollment of more than 450 patients at 45 sites worldwide has been recently completed.
Another study involving the epimacular brachytherapy procedure is focusing on patients who appear to require persistent injections of anti-VEGF therapies in order to maintain an adequate response. A total of 50 patients were enrolled in this Phase I/II trial who had received a minimum of eight injections during the prior 12 months or six injections in the prior six months. Preliminary analysis of 16 patients suggested that a single treatment with epimacular brachytherapy could further improve visual acuity while decreasing the number of required anti-VEGF injections. An improvement in visual acuity was seen in 63 percent of these patients, with 50 percent gaining five or more letters of visual acuity by six months post-procedure.
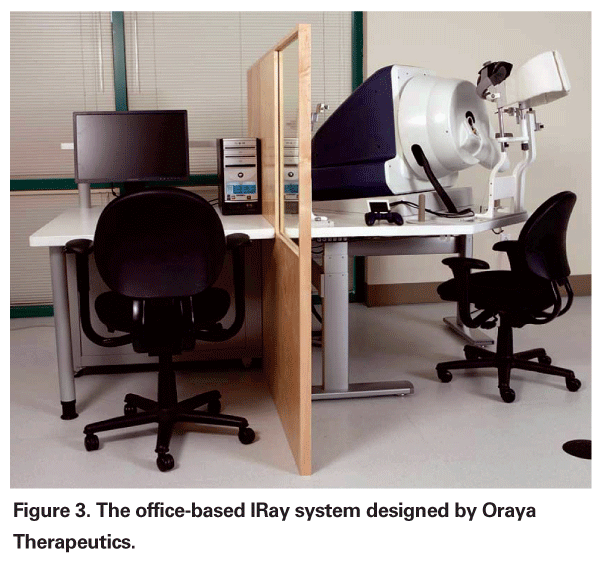
External Beam Radiotherapy
An office-based external beam radiotherapy treatment known as the IRay system (Oraya Therapeutics,

Each treatment involves two to three fractionated doses of 8 Gy for a total of 16 to 24 Gy, similar to the NeoVista study. One beam will enter approximately at mid-quadrant inferonasally; the second beam will enter inferiorly at 6 o'clock; and the third beam will enter approximately at mid-quadrant inferotemporally. Based on computational modeling techniques, the mean absorbed dose should be approximately 0.125 Gy to the lens, 0.219 Gy to the optic nerve, and 0.012 Gy to the brain.28,29 The total dose is expected to be comparable to a diagnostic head radiograph and tenfold less than a head CT scan.
The benefits of an office-based procedure are numerous. Oraya Thera-peutics estimates that each procedure should take only 15 to 20 minutes to complete. Since it is non-invasive, there is no additional risk of infection, hemorrhage, retinal tears or retinal detachment. The rate of cataract formation should also not be accelerated since the beam path with the IRay system avoids the lens and by computational modeling is well below the threshold for inducing cataracts.
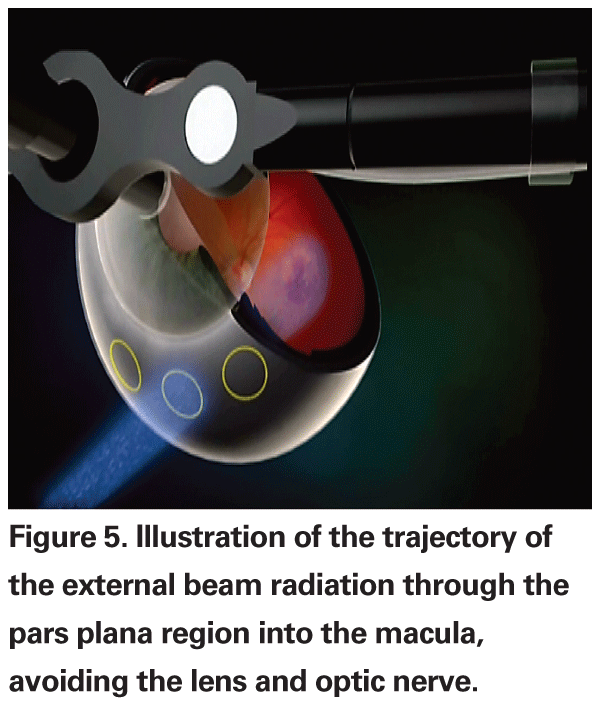
However, to date, no clinical data has been published about the IRay system. A feasibility trial has shown that the device is well-tolerated with only topical anesthesia and has not been associated with any short-term complications. Recently, a masked, sham-controlled study of the device delivering 16 Gy or 24 Gy compared to sham was announced. The clinical trial will be conducted in
Future Role of Radiation Therapy
The future role of radiation therapy for AMD is contingent on the results of larger, Phase III randomized clinical trials. Based on the initial trials with the strontium-90 applicator, radiation therapy holds the greatest promise as part of a combined approach with anti-VEGF injections. If radiation therapy can be proven to decrease the number of required anti-VEGF injections while still maintaining results similar to the clinical trials with monthly intravitreal Lucentis injections, then this combination could significantly reduce the tremendous cost and projected treatment burden associated with neovascular AMD. In addition, the burden to patients in terms of visits and anxiety over injections could be minimized as well.
Dr. Hsu is a member of the Wills Eye Institute Retina Service, a clinical instructor of ophthalmology at
1. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432-1444.
2. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.
3. Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol 2008;145:239-248.
4. Krishnan L, Krishnan EC, Jewell WR. Immediate effect of irradiation on microvasculature. Int J Radiat Oncol Biol Phys 1988;15:1447-1450.
5. Chakravarthy U, Gardiner TA, Archer DB, Maguire JF. A light microscopic and autoradiographic study of non-irradiated and irradiated ocular wounds. Curr Eye Res 1989;8:337-348.
6. Mooteri SN, Podolski JL, Drab EA, Saclarides TJ, Onoda JM, et al. WR-1065 and radioprotection of vascular endothelial cells, II: morphology. Radiat Res 1996;145:217-224.
7. Rosander K, Zackrisson B. DNA damage in human endothelial cells after irradiation in anoxia. Acta Oncol 1995; 34:111-116.
8. Rubin DB, Drab EA, Kang HJ, Baumann FE, Blazek ER. WR-1065 and radioprotection of vascular endothelial cells, I: cell proliferation, DNA synthesis, and damage. Radiat Res 1996;145:210-216.
9. Verheif M, Koomen GCM, van Mourik JA, Dewitt L. Radiation reduces the cyclooxygenase activity in cultured human endothelial cells at low doses. Prostaglandins 1994;48:351-366.
10. Hosoi Y, Yamamoto M, Ono T, Sakamoto K. Prostacyclin production in cultured endothelial cells is highly sensitive to low doses of ionizing radiation. Int J Radiat Biol 1993;63:631-638.
11. Hallahan D, Clark ET, Kuchibhotla J, Gewertz BL, Collins T. E-selectin gene induction by ionizing radiation is independent of cytokine induction. Biochem Biophys Res Commun 1995;217:784-795.
12. Eissner G, Kohluhuber F, Grell M, Ueffing M, Scheurich P, et al. Critical involvement of transmembrane tumor necrosis factor-alpha endothelial programmed cell death mediated by ionizing radiation and bacterial endotoxin. Blood 1995;86:4184-4193.
13. Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy-clinical histopathological, ultrastructural and experimental correlations. Eye 1991;5:239-251.
14. Maguire AM, Schachat AP. Radiation retinopathy. In: Retina.
15. The AMDRT Research Group. The Age-Related Macular Degeneration Radiotherapy Trial (AMDRT): One year results from a pilot study. Am J Ophthalmol 2004;138:818-828.
16. Valmaggia C, Ries G, Ballinari P. Radiotherapy for subfoveal choroidal neovascularization in age-related macular degeneration: A randomized clinical trial. Am J Ophthalmol 2002;133:521-529.
17. Hoeller U, Fuisting B, Schwartz R, Roeper B, Richard G, Alberti W. Results of radiotherapy of subfoveal neovascularization with 16 and 20 Gy. Eye 2005;19:1151-1156.
18. Marcus DM, Sheils WC, Johnson MH, McIntosh SB, Letbach DB, et al. External bean irradiation of subfoveal choroidal neovascularization complicating age-related macular degeneration. Arch Ophthalmol 2001;119:171-180.
19. Hart PM, Chakravarthy U, Mackenzie G, Chisholm IH, Bird AC, et al. Visual outcomes in the subfoveal radiotherapy study. Arch Ophthalmol 2002;120:1029-1038.
20. Sivagnanavel V, Evans JR, Ockrim Z, Chong V. Radiotherapy for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No:CD004004. DOI: 10.1001/14651858.CD004004.pub2.
21. Avila MP, Farah ME, Santos A, Kapran Z, Duprat JP, et al. Twelve-month safety and visual results from a feasibility study of intraocular, epiretinal radiation therapy for the treatment of subfoveal CNV secondary to AMD. Retina 2009;29:157-169.
22. Ikeda T, Sawa H, Koizumi K, Yasuhara T, Yamasaki T. Pars plana vitrectomy for regression of choroidal neovascularization with age-related macular degeneration. Acta Ophthalmol Scand 2000;78:460-464.
23. Stefansson E, Landers MB, Wolbarsht ML. Vitrectomy, lensectomy, and ocular oxygenation. Retina 1982;2:159-166.
24. Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in squamous cell carcinoma of the head and neck. Radiother Oncol 1996;41:31-39.
25. Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Research 1996;56:941-943.
26. Avila MP, Farah ME, Santos A, Duprat JP, Woodward BW, et al. Twelve-month short-term safety and visual-acuity results from a multicentre prospective study of epiretinal strontium-90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularisation secondary to age-related macular degeneration. Br J Ophthalmol 2009;93:305-309.
27. Lee C, Chell E, Gertner M, Hansen S, Howell RW, et al. Dosimetry characterization of a multibeam radiotherapy treatment for age-related macular degeneration. Med Phys 2008;35:5151-5160.
28. Girkin CA, Comey CH, Lunsford LD, Goodman ML, Kline LB. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology 1997;104:1634-1643.
29. Hanlon J, Lee C, Chell E, Gertner M, Hansen S, et al. Kilovoltage stereotactic radiosurgery for age-related macular degeneration: Assessment of optic nerve dose and patient effective dose. Med Phys 2009;36:3671-3681.



