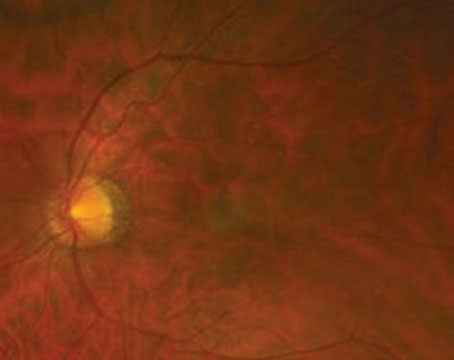
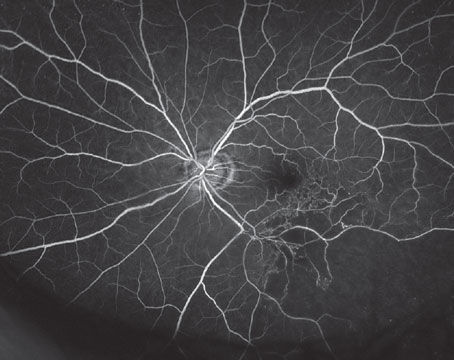
Proliferative vitreoretinopathy has been referred to by various nomenclatures.1 It was first designated as massive vitreous retraction, based on its ophthalmoscopic appearance (See Figure 1) and then massive periretinal proliferation,1 based on its histologic findings (See Figure 2). The 1983 Retina Society classification of PVR was amended in 1989 by the Silicone Oil Study Group2 to its current designation: proliferative, to denote proliferation of retinal pigment epithelium cells, as well as fibrous metaplasia of cells derived from the RPE and glial cells; and vitreoretinopathy, to refer to the structures affected (vitreous and retina). This classification was designed to document the extent and anatomic distribution of PVR present preoperatively and to help standardize the surgical treatment. (The Silicone Oil Study was a randomized, multicenter trial that investigated the surgical management of retinal detachment with PVR.)
Natural History and Etiology
PVR is the most common complication of retinal detachment and occurs in approximately 5 to 10 percent of patients following primary retinal detachment repair. It may also occur in patients with chronic untreated RD.

The etiology of PVR starts at the time of the retinal tear and retinal detachment, at which time RPE cells under the retina migrate through the retinal tear and enter the vitreous cavity. These cells then proliferate in the vitreous and on the surface of the retina causing fibrous tissue formation. PVR can occur in any location of the vitreous and retina. However, it is more commonly seen in the inferior retina due to gravity, as the proliferative cells and mediators settle inferiorly.
The processes of cellular proliferation followed by membrane contraction that constitute PVR usually occur within three months of primary RD repair. Similarly, the risk of recurrent RD following initial treatment of PVR is also greatest about two months after surgery.
Associated Risk Factors
Risks factors that can enhance the likelihood of the development of PVR include: aphakia; multiple retinal tears; large retinal detachment involving greater than two quadrants of the eye; pre- or post-choriodal detachment; vitreous hemorrhage; multiple surgical interventions; and trauma.
Classification of PVR
Classification of PVR uses both grade (severity of disease) and contraction type/location (focal, diffuse, subretinal, circumferential, anterior loop/anterior or posterior to the equator) to describe the status of the retina.2
There are two main classifications of PVR: the Retina Society Terminology Committee (four grades: A,B,C and D) and the Silicone Oil Study designations (three grades: A, B and C). (See Table 1.)
Management of PVR-associated RD
The treatment of PVR is surgical. The goal of surgery for PVR is to reattach the retina by identifying all retinal breaks and relieving all significant vitreoretinal traction.3 Sometimes scleral buckle placement only (circumferential band with high segmental sponge that closes the breaks) and subretinal fluid drainage may successfully reattach a retina with PVR. More often than not, however, the addition of vitrectomy and membrane peeling is required.
The success of vitrectomy in the treatment of PVR is predicated on important intraoperative goals. Firstly, a meticulous pars plana vitrectomy and shaving of the vitreous base is performed. This is followed by a 360-degree, scleral-depressed peripheral retinal examination using a wide-field viewing system to identify and then diathermize all the retinal breaks.

The sine qua non of PVR surgery is complete membrane peeling to relieve all tangential retinal traction, thus allowing the retina to conform to the RPE/choroid. We believe that using a bimanual technique (lighted pick and forceps) allows for the safest and most effective method for peeling membranes off the retina. The most critical decision point in PVR surgery is the careful evaluation of the retina for persistent retinal traction after membrane peeling is complete. In cases where there is persistent traction despite complete membrane peeling (due to subretinal or intrinsic retinal contraction), a sufficiently large relaxing retinotomy must be performed to relieve the persistant retinal traction (See Figure 4). The retina is then temporarily flattened under air (draining through a drainage retinotomy or at the edge of the relaxing retinotomy) or perfluorocarbon oil.
Next, all of the retinal breaks and edges of any retinotomies (drainage or relaxing) are treated confluently with laser. Excessive scleral indentation,4 laser retinopexy or cryotherapy5 should be avoided as they can release viable RPE cells and stimulate further cellular proliferation. The intraocular gas C3F8 and silicone oil6 (1,000 cs or 5,000 cs) are typically used to provide extended endoretinal tamponade. Per the Silicone Oil Study, C3F8 gas is superior to SF6 gas, as it has a longer duration of about six to eight weeks compared to three to four weeks with SF6. And C3F8 gas tamponade was found to be equally effective compared to silicone oil tamponade.

Silicone oil removal is typically done about three to six months after surgery and is associated with a 20-percent risk of retinal redetachment.7
Note that phakic patients typically will develop a cataract during or following vitrectomy surgery with gas or silicone oil tamponade. In most phakic cases, we often will perform pars plana lensectomy or, more commonly, combined cataract extraction and retinal detachment surgery repair.
In regards to postoperative care, it is important to evaluate the patient for retinal attachment, size of the intraocular tamponade, intraocular pressure, anterior chamber depth, endophthalmitis and hemorrhage, and to address correct head positioning to maximize the effectiveness of retinal tamponade agent.

Medications
Medications may be useful in the management of patients undergoing surgical repair of a RD associated with PVR.8
• Steroids (topical subconjunctival/posterior sub-Tenon's/oral prednisone) have been found to be effective in reducing postoperative re-proliferation if given prior to surgery in an animal model of PVR. Recommended dose is 60 to 80 mg three days prior to surgery followed by a quick taper over two weeks.
• Minocycline (neuroprotective agent and glial cell inhibitor) has been found to inhibit photoreceptor apoptosis and glial cell proliferation in an animal model of RD. Recommended dosage: 100 mg one tablet
• Other agents under investigation:
— 5 Fluorouracil and Low-Weight Molecular Heparin (LWMH) infusion.9
— VitrenAse (Vit 100), an antiproliferative agent that halts growth of PVR membranes by stopping the formation of proteins necessary to make new scar tissue.
— Danunrobicin (acts as a topiosomerase inhibitor).10
— Gene therapy.
— Densiron (Medicel,

Future Avenues for PVR
PVR is a condition that has potentially catastrophic implications for patients' visual recovery after retinal detachment surgery. Today, with enhanced surgical techniques and smaller gauge technology less trauma is done to the retinal tissues in treating retinal detachments and in addressing PVR. Developing a better understanding of the pathophysiology of wound healing and cellular proliferation will uncover additional targets for treatment and prevention of PVR. Some of these targets include hepatocyte growth factor and connective tissue growth factor. Generating medications to target these and other PVR-promoting substances12 and identifying pathways which trigger PVR and intercepting these prior to the development of PVR are critical to reducing the rate of this dreaded complication.

Dr. Arroyo is an assistant professor of ophthalmology at
1. Machemer, R Pathogenesis and classification of massive periretinal proliferation. Br J Ophthalmol 1978;62:737-47.
2. Lean JS, Stern WH, et al. Classification of proliferative vitreoretinopathy used in the silicone study. The Silicone Study Group. Ophthalmology 1989;96:765-771.
3. Machemer R, Laqua H. A logical approach to the treatment of massive periretinal proliferation. Ophthalmology 1978;85:584-93.
4. Singh AK, Michels RG, Glaser BM. Scleral indentation following cryotherapy and repeat cryotherapy enhance release of viable retinal pigment epithelial cells. Retina 1986;6:176-8.
5. Campochiaro PA, Kaden IH, Vidaurri-Leal J, Glaser BM. Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells. Arch Ophthalmol 1985;103:434-6.
6. Alexander P, Prasad R, Ang A, Poulson AV, Scott JD, Snead MP. Prevention and control of proliferative vitreoretinopathy: Primary retinal detachment surgery using silicone oil as a planned two-stage procedure in high-risk cases. Eye 2008 Jun;22(6):815-8. Epub 2007 Feb 2.
7. Lam RF, Cheung BT, et al. Retinal redetachment after silicone oil removal in proliferative vitreoretinopathy: A prognostic factor analysis. Am J Ophthalmol 2008;145:527-533.
8. Sun JK, Arroyo JG. Adjunctive therapies for proliferative vitreoretinopathy. Int Clinics of Ophthalmol 2004;44:1-10.
9. Garcia RA, Sanchez JG, Arevalo JF. Combined 5-fluorouracil, low-molecular weight heparin, and silicone oil in the management of complicated retinal detachment with proliferative vitreoretinopathy grade C. Ophthalmic Surg Lasers Imaging 2007;38:276-82.
10. Shinohara K, Tanaka M, Sakuma T, Kobayashi Y. Efficacy of daunorubicin encapsulated in liposome for the treatment of proliferative vitreoretinopathy. Ophthalmic Surg Lasers Imaging 2003;34:299-305.
11. Lim BL, Vote B. Deniron intraocular tamponade: A case series. Clin Experiment Ophthalmol 2008;36:261-4.
12. Yu J, Liu F, Cui SJ, et al. Vitreous proteomic analysis of proliferative vitreoretinopathy. Proteomics 2008;8:3667-78.