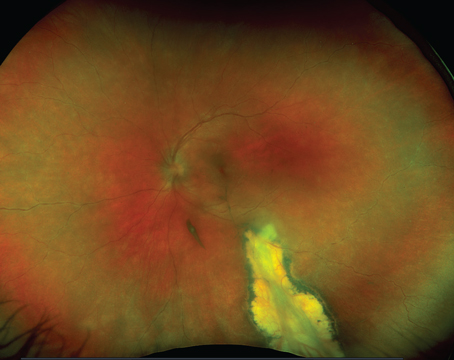
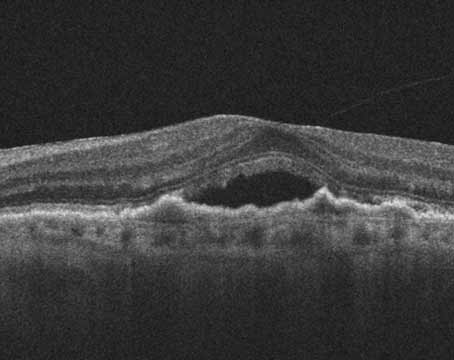
Age-related macular degeneration is a serious disease with the potential for increasingly significant morbidity as our population ages. Within the scope of AMD, neovascular disease has potentially devastating visual consequences. Fortunately, as our understanding of neovascular AMD improves, so do our options for treatment. Vascular endothelial growth factor has emerged as one of the primary mediators accounting not only for angiogenesis, but vascular permeability. Now, with the results of prospective randomized clinical trials, anti-VEGF therapy remains the mainstay of treatment for neovascular AMD.
For almost two decades, laser photocoagulation was the only evidence-based treatment, but it merely limited the spread of neovascular AMD until the advent of more focal photodynamic therapy. Just a few years later, the Food and Drug Administration approved the first anti-VEGF agent in ocular disease, headlining a paradigm shift in the treatment of neovascular disease of the eye. For a large percentage of patients with wet AMD, science has significantly improved their lives; however, amidst the new wave of solutions the problem of anti-VEGF non-responders emerges. This article aims to review anti-VEGF therapy for neovascular AMD and shed light on how we can tackle treatment failures.
Angiogenesis—VEGF and Beyond
Mouse models demonstrate that VEGF is critical in regulating angiogenesis in development of the circulatory system as even homozygous deletions are incompatible with life. VEGF-A, in particular, can be singled out as one of the primary proteins required for proper vessel formation. 1-2 Its expression is upregulated especially in response to hypoxia. In solid tumors, VEGF-A is highly expressed as compared to normal tissue and increases vascular permeability, growth, and metastatic potential.
While there is growing knowledge regarding VEGF and its pro-angiogenic properties, little is known about the natural negative regulators of angiogenesis. The tumor literature describes large glycoproteins produced by epithelial cells, including thrombospondin, as well as endothelial derived proteins like vasohibin that play vital roles in the regulation of angiogenesis. 3-4 Some of these endogenous proteins have been shown to inhibit angiogenesis associated with tumor growth, however their role in intraocular disease has yet to be determined.
Our current understanding of neovascular AMD is that neovascular growth and leakage are the main components of the disease process. Clinical studies have strengthened the connection between VEGF and neovascular AMD. Increased levels have been reported in the retinal pigment epithelium and in choroidal neovascular membranes from patients with neovascular AMD. With angiogenesis as our backdrop, we have set the stage to target therapy for neovascular AMD.
Anti-VEGF Therapies
The case for targeted anti-VEGF therapy for neovascular AMD has been proven in multiple prospective trials. Pegaptanib is an RNA aptamer that targets the main subtype of VEGF-A, VEGF165. In the Phase III VEGF Inhibition Study In Ocular Neovascularization (VISION), intravitreal pegaptanib slowed visual loss in neovascular AMD and 6 percent of pegaptanib treated patients gained > 15 letters compared to 2 percent in the control group. 5
Only a few years later, data from the
Further, bevacizumab (Avastin) is a full-length humanized monoclonal antibody to VEGF, which is also being used for the treatment of neovascular AMD. While it is only utilized off-label, there are non-randomized studies that demonstrate its effectiveness in the treatment of exudative AMD.
Treatment Failures, Semantics Aside
No matter how you define it, treatment failures with anti-VEGF agents exist. Prior studies deemed treatment success as stabilization of vision loss. As standards for treatment success are raised, more attention should be focused on visual acuity gains as the primary endpoint. With this in mind, the proportion of patients gaining vision after treatment with anti-VEGF agents is not as impressive. Despite treatment success compared to PDT, the VISION study still reported that around 40 percent of patients treated with pegaptanib lost at least 15 letters from their pretreatment vision 5. Of patients treated with ranibizumab in the
Recently Anja Lux, MD, and colleagues set out to determine why almost half of patients treated with anti-VEGF agents do not improve.8 They report that 45 percent of patients with CNV secondary to AMD treated with bevacizumab were "non-responders." This alarmingly high rate is, in part, a product of their patient pool (36 percent had prior therapy) and their definition of non-responders: a reduction or no change in visual acuity or reading ability. Nevertheless, on a case by case basis, not a single study reports universal response to anti-VEGF therapies.
Poor Response or Poorly Directed?
Evidence regarding anti-angiogenesis therapy resistance in the tumor literature is growing. There are reports that resistance to anti-VEGF-A antibodies may be secondary to regulatory feedback loops in the Golgi apparatus of tumor cells. Solid tumor cells may also develop resistance to hypoxia and become less dependent on angiogenesis or develop more mature vessels through remodeling that result in stable vascularization that is less responsive to anti-angiogenic therapy. These VEGF-A-centric theories address only one side of the problem, forgetting the influence of VEGF-independent factors.
The focus on VEGF-dependent angiogenesis has cast a shadow on VEGF-independent pathways that may contribute to anti-VEGF therapy resistance. Although the numerous molecular signaling pathways are beyond the scope of this article, signaling systems like the angiopoietin-tie are known pro-angiogenic mediators that contribute to vessel formation and stabilization.9-10 Even lymphangiogenic mediators may play a role as VEGF-C and VEGF-D bind to VEGF receptors that are involved in angiogenesis. The role of these additional mediators is largely unknown but currently being investigated in relation to ocular neovascularization.
Shlomit Schaal, MD, PhD, and colleagues recently explored the concept of tachyphylaxis associated with repeat bevacizumab injections for neovascular AMD. They found that roughly three injections of bevacizumab were required to decrease the initial efficacy by 50 percent, however combining the injections with preservative-free triamcinolone increased the number of injections to five before the response to therapy was less effective.11 They postulate that tachyphylaxis may be partially alleviated by combining medical therapies with different mechanisms of action. Similarly, Ruslan Hluschchuk, MD, et al report synergistic action of VEGF-inhibitors with platelet derived growth factor inhibitors - making the case for multi-modal therapies when trying to control vascularization. 12
A retrospective review from the National Eye Institute found that it took between five and 10 injections of bevacizumab before tachyphylaxis occurred.13 They postulate that even increasing the dose does not counteract the eventual lack of response to therapy. Citing molecular studies, they propose that macrophages may be responsible for tachyphylaxis as they proliferate and increase VEGF production in response to anti-VEGF therapies.
CNV lesion composition may also be attributing to treatment failures. Polypoidal choroidal vasculopathy (PCV) has been suggested as a variant of neovascular AMD. Compiling results from several case series, PCV has a prevalence of 8 percent to 13 percent among whites with neovascular AMD. In a retrospective case series, 12 eyes deemed refractory to anti-VEGF therapy in neovascular AMD were found to have PCV thought to be responsible to lack of treatment response. Nine out of 12 patients were treated with combination PDT and anti-VEGF therapy with eight of those showing complete response to treatment as determined by indocyanine green angiography or optical coherence tomography.14 However, visual acuity was the same or worse in nine out of 12 patients blurring what is considered treatment success.
Retinal angiomatous proliferation (RAP) is another recognized lesion that responds differently than the typical CNV seen in wet AMD. Several published reports have demonstrated beneficial results in treating these lesions with anti-VEGF therapy alone. There is also evidence that PDT in combination with intravitreal triamcinolone as well as PDT with anti-VEGF therapy is effective for RAP. Alexandros Rouvas, MD, and colleagues report positive results in all groups of a small prospective series comparing ranibizumab, PDT with IVT, and PDT with ranibizumab.15 They ultimately concluded that PDT with IVT may be the best option for RAP as there was a trend toward better visual acuity, anatomical improvement, and fewer injections required based on their retreatment algorithm.
Another theory as to why there are treatment failures requires an understanding of capillary vessel stabilization. Capillary walls differ from arteries and veins in that they are made of a monolayer of pericytes instead of multiple layers of smooth muscles. Capillary angiogenesis is a VEGF-dependent process until the walls of the capillary are deemed mature. Once lined with pericytes, capillary regression becomes a VEGF-independent process. In fact, some studies show that pericytes actually stabilize the growth of these capillaries. The interaction between angiopoietin and tie expressed on the surface of endothelial cells is thought to be involved in vessel stabilization through pericyte and endothelial cell interaction.16 This is essentially a VEGF-independent process that, in theory, could lead to aberrant angiogenesis and stabilization even in the presence of anti-VEGF antibodies.
A Combination of Monotherapies
The initial trials using anti-VEGF monotherapy studied it as a regimented treatment strategy that is difficult to emulate in today's clinical setting. Since most practitioners do not follow the monthly injection protocols, the question becomes a matter of when one decides to classify a patient as a non-responder. Is a patient a non-responder after failure to improve with only one injection or does the patient need multiple treatments before alternative therapies should be tried? If so, for how long and after how many injections can one safely say that anti-VEGF-A therapy alone is inadequate for this said patient? These are questions with no clear answers, however we can apply what knowledge we have to tailor therapy for those who fall outside of our simple treatment strategy.
PrONTO was an excellent study using OCT to guide therapy for injections of ranibizumab, achieving results comparable to the large prospective trials but with less than half the required number of injections.17 Using anatomical markers as an index for treatment response can help direct therapy.
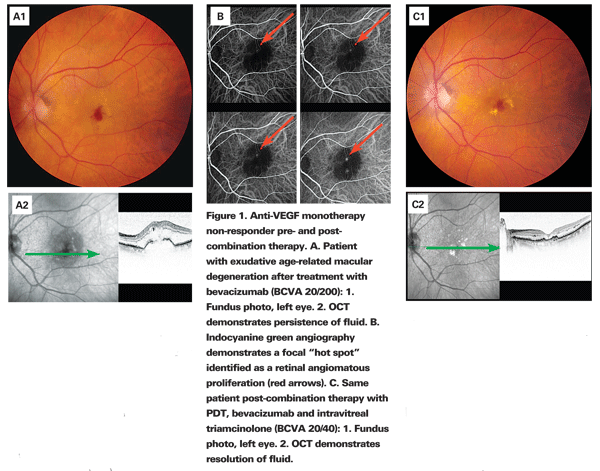
Considering lesion composition is also an important factor in directing treatment. As discussed above, PCV and RAP are more difficult to treat as they behave differently than classic CNV. Fluorescein angiography can help distinguish classic from occult CNV in wet AMD but provides little information beyond this. ICGA is an accepted way of improving the imaging of occult CNV seen by FA. Both PCV and RAP can be accurately diagnosed with ICGA and are seen as a "hot spot" with intense hyperfluorescence in the mid to late phase of the study.18 As discussed earlier, these lesions could account for anti-VEGF treatment failures, however, with proper identification; the practitioner may opt to apply combination therapy knowing that monotherapy response can be poor.
In light of what we know regarding angiogenesis including VEGF and non-VEGF mediated pathways, it comes as no surprise that mono-therapy is not universally effective. Figure 1 demonstrates a representative case of a patient previously treated with bevacizumab with persistent leakage. Visual acuity was 20/200 with anatomical correlation on OCT. After combination therapy with PDT, bevacizumab, and intravitreal triamcinolone, there was improvement in visual acuity to 20/40 with resolution of fluid as demonstrated by OCT.
Verteporfin and anti-VEGF combination treatment strategies are being extensively studied and show promise in anatomical and functional improvement in wet AMD. The FOCUS study was the first to investigate combination therapy with verteporfin treatment seven days before ranibizumab or sham injection. After two years, patients with combination therapy gained 4.6 letters compared to 7.8 lost letters in verteporfin therapy alone.19
Another prospective series reports that therapy with verteporfin, bevacizumab, and corticosteroids can obtain functional and anatomic improvement suggesting a potential role for triple therapy in neovascular AMD.20 Here, 104 patients with all types of CNV due to AMD received a reduced light dose of verteporfin therapy (42J/cm2) followed by intravitreal dexamethasone and bevacizumab 16 hours later. After a mean follow up of 40 weeks, only five patients needed repeat triple therapy and 18 needed an additional bevacizumab injection. Visual acuity improved 1.8 lines and retinal thickness decreased by 182µm.
Although no evidence exists for when to use multimodal therapy, there are instances in which combination therapy may be advantageous over monotherapy. Patients with the following may be candidates: growing CNV membranes after three to four anti-VEGF treatments; persistent thickening after six anti-VEGF therapies with leakage on FA; classic CNV with acuity < 20/50; or occult leakage with evidence of active disease especially secondary to PCV or RAP. A suggested same-day treatment protocol for patients who failed previous treatment for wet AMD is supported by data presented at the 2009 meeting of the Association for Research in Vision and Ophthalmology (Sang D, et al. IOVS 2009;50:ARVO E-Abstract 1927). Forty patients were treated with reduced duration PDT (42 secs., 600mW/cm2) followed by 500 µg of intravitreal dexamethasone and 1.25 mg of intravitreal bevacizumab one hour after PDT. These patients had failed prior anti-VEGF monotherapy, with or without previous history of PDT or transpupillary thermal therapy, and with or without previous steroid injection. There was no association with lack of response to this triple therapy with lesion type and furthermore 57 percent of the eyes had increased Snellen visual acuity with 80 percent showing decreased OCT thickness.
The days of targeted therapies have blossomed and will only continue as the molecular mechanisms of eye disease are revealed. With more and more injections, we will discover more and more non-responders. These non-responders will need alternative treatment strategies to break out of the cycle of monthly injections with the same stagnant results. Now as data from prospective studies emerges, the hypothesis that combination therapy for this disease may be superior to single agents in patients who fail to respond to anti-VEGF treatment alone may soon be answered. In some patients initial combination therapy for RAP or PCV disease may be warranted given the improved response they have for PDT, anti-VEGF and steroid therapy. We expect that multimodal therapy for neovascular AMD will certainly have its place in clinical practice.
Dr. Sheybani is a resident in ophthalmology and Dr. Almony a vitreoretinal fellow at the Barnes Retina Institute. Dr. Binder and Dr. Shah practice at Barnes and Dr. Shah is also a clinical associate professor with Washington University Department of Ophthalmology and Visual Sciences.
1. Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single vegf allele. Nature 1996;380(6573):435-439.
2. Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380(6573):439-442.
3. Dipietro L. Thrombospondin as a regulator of angiogenesis. EXS 1997;79:295-314.
4. Watanabe K, Hasegawa Y, Yamashita H, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest 2004;114(7):898-907.
5. Gragoudas E, Adamis A, Cunningham Jr. E, et al. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351(27):2805-2816.
6. Rosenfeld P, Brown D, Heier J, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355(14):1419-1431.
7. Brown D, Kaiser P, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355(14):1432-1444.
8. Lux A, Llacer H, Heussen F, et al. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol 2007;91(10):1318-1322.
9. Cho C, Kim K, Byun J, et al. Long-term and sustained comp-ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ Res 2005;97(1):86-94.
10. Uemura A, Ogawa M, Hirashima M, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest 2002;110(11):1619-1628.
11. Schaal S, Kaplan H, Tezel T. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration? Ophthalmology 2008;115:2199-2205.
12. Hlushchuk R, Baum O, Gruber G, et al. The synergistic action of a VEGF-receptor tyrosine-kinase inhibitor and a sensitizing PGDF-receptor blocker depends upon the stage of vascular maturation. Microcirculation 2007;14(8):813-825.
13. Forooghian F, Cukras C, Meyerle C, et al. Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina 2009;29:723-731.
14. Cho M, Barbazetto I, Freund K. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 2009;148:70-78 e71.
15. Rouvas A, Papakostas T, Vavvas D, et al. Intravitreal ranibizumab, intravitreal ranibizumab with PDT, and intravitreal triamcinolone with PDT for the treatment of retinal angiomatous proliferation: A prospective study. Retina 2009;29:536-544.
16. Holash J, Wiegand S, Yancopoulos G. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 1999;18(38):5356-5362.
17. Fung A, Lalwani G, Rosenfeld P, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007;143:566-583.
18. Fernandes L, Freund K, Yannuzzi L, et al. The nature of focal areas of hyperfluorescence or hot spots imaged with indocyanine green angiography. Retina 2002;22:557-568.
19. Heier J, Boyer D, Ciulla T, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: Year 1 results of the focus study. Arch Ophthalmol 2006;124:1532-1542.
20. Augustin A, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: Verteporfin PDT, bevacizumab, and dexamethasone. Retina 2007;27:133-140