Bausch Health Companies, Bausch + Lomb and Eton Pharmaceuticals announced that the FDA has approved Alaway Preservative Free (ketotifen fumarate ophthalmic solution 0.035%), antihistamine eye drops (EM-100), as the first over-the-counter, preservative-free formulation eye drop approved to temporarily relieve itchy eyes due to pollen, ragweed, grass, animal hair and dander. The company says it avoided using preservatives in the formulation because common preservatives can cause allergic reactions in some people that may lead to ocular redness, irritation, itching or tearing. B+L says Alaway Preservative Free is formulated to relieve itch within minutes and can provide up to 12 hours of eye itch relief with one dose. For information, visit bausch.com.
Fundus Imaging Anywhere
If you regularly visit patients in nursing homes, need to get a fundus image in the parking lot to follow your COVID-19 protocols or participate in screening events, Volk Optical says its new VistaView portable mydriatic retinal camera may be worth a look.
The VistaView integrates Volk’s high-resolution, all-glass, double-aspheric optics with an intuitive digital platform to capture sharp, wide-field fundus images while managing patient data right on the device, the company says.
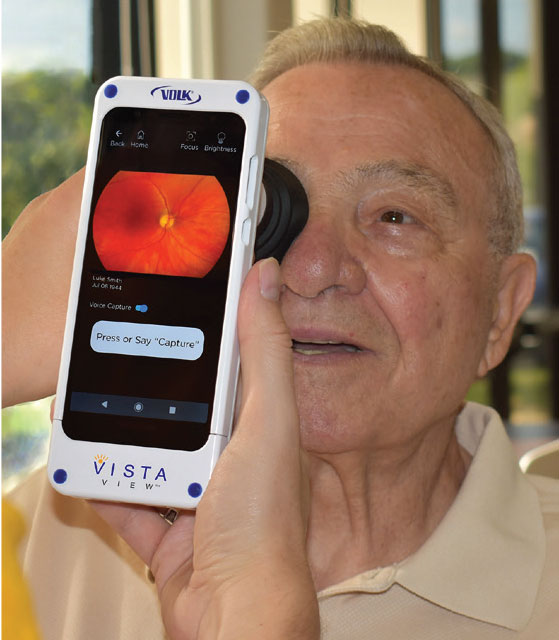 |
Volk says the VistaView is an all-in-one device that offers the widest field of view of any mydriatic fundus camera in its class at 55 degrees. The onboard application includes features such as image review, enhancement and analysis; and instant password-protected report generation and transmission.
A voice-capture option leaves both hands free to position the device for the best quality images, the company adds. The device has both autofocus and manual focus modes, as well as an illumination adjustment to help make photophobic patients more comfortable. Password protected reports and DICOM images are easily shared or exported for billing, consultation and referral, Volk says.
For information, visit volk.com/vista.
Glide into Easier Glaucoma Surgery
If you routinely perform the Kahook Dual Blade glaucoma procedure, or are considering adding it to your arsenal, New World Medical recently announced a new instrument that might make it easier for you.
The company says that the new KDB Glide is designed to give surgeons a refined, precise experience performing excisional goniotomy. New World Medical says the new device improves on the successful Kahook Dual Blade technology by adding new features: A rounded heel, tapered sides and a smaller footplate deliver optimal interface with the canal of Schlemm, permitting a precise excision with the instrument’s dual blades, even in variable anatomy.
If you’re new to excisional goniotomy procedures for the treatment of glaucoma, they involve removing a segment of the diseased trabecular meshwork, which surgeons say facilitates the flow of aqueous into collector channels of the eye, thus alleviating intraocular pressure. For information, visit newworldmedical.com.



