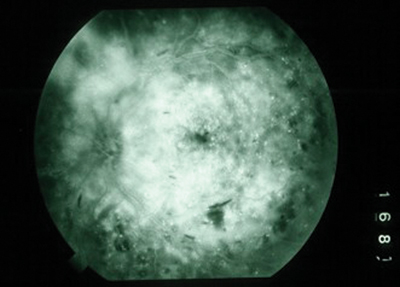
 |
| Figure 1. Fluorescein angiogram demonstrating features of refractory diffuse CSDME: broad, diffuse, petalloid hyperfluorescence with few microaneurysms. |
Why Vitrectomy?
The role of the vitreous in the development or progression of macular edema has been described for over two decades. Vitrectomy has been investigated as a treatment alternative for refractory CSDME for approximately 15 years. Initially, Charles Schepens suggested that vitreous traction may play a role in the cystoid macular edema associated with retinitis pigmentosa, aphakia, and uveitis.3
Later, F.P. Nasrallah suggested a possible role for the vitreous in the development of CSDME when he reported that eyes with CSDME were less likely to have a posterior vitreous detachment (PVD) than those without CSDME.4 The spontaneous resolution of CSDME in 55 percent of eyes that developed a PVD versus 25 percent of eyes that did not was also reported.5 Beyond such published reports, there has long been anecdotal evidence of resolution of CSDME in eyes after vitrectomy for complications of proliferative diabetic retinopathy.
Shortly after the description of the successful manipulation of the vitreomacular interface to close macular holes,6 reports began to suggest that the vitreomacular interface could be surgically manipulated to effectively treat CSDME as well. Visual acuity was reported to improve following vitrectomy and membrane peeling of a visibly taut, opacified posterior hyaloid in eyes with diffuse CSDME.7
Since then, multiple authors have retrospectively reported that vitrectomy and separation of both visibly taut and presumably invisibly taut, attached posterior hyaloid membranes resulted in resolution of CSDME in 43 to 100 percent of eyes and VA improvement of >2 lines in 38 to 92 percent of eyes.8-19 In a small, prospective, controlled trial, researchers demonstrated statistically significant VA improvement and decreased macular thickness (by OCT) in vitrectomized eyes compared to a control group of observed matched fellow eyes.20 In most eyes, resolution of CSDME has been shown to occur between three and six months postoperatively, though improvement has been seen to begin earlier (See Figures 2 and 3).11,13
Vitrectomy has also been described to benefit eyes with end-stage CSDME. Following vitrectomy and subretinal mechanical removal of massive subfoveal hard exudates, one report showed VA improvement in five of seven eyes while CSDME resolution was seen in all eyes despite macular holes occurring in four of the eyes.21 Long-term results following this procedure were published by another group, showing that VA improvement was maintained in 54 percent of eyes at one year and 38 percent at three to four years postoperatively.22 In our current series, with a mean follow-up of 16 months, we have seen VA improvement of >2 Snellen lines in 10 of 16 eyes and CSDME resolution or reduction in all eyes following vitrectomy and submacular irrigation of hard exudates (without mechanical forceps extraction).
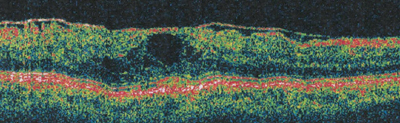 |
| Figure 2a. Pre-vitrectomy OCT in an eye with visibly opacified and taut posterior hyaloidal traction, VA = 20/200. |
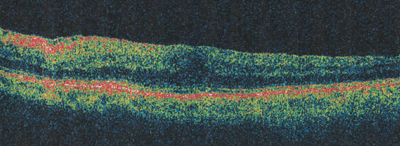 |
| Figure 2b. Three-month post-vitrectomy OCT, VA = 20/60. |
Nearly every published series that has evaluated vitrectomy for diffuse, refractory CSDME has demonstrated significant VA improvement when compared to observation and macular laser. Most of them are small, single-center, retrospective, and nonrandomized, and have short follow-up periods that include eyes with nonstandardized VAs and variable inclusion criteria. Nonetheless, the outcomes from these studies are consistent: They strongly suggest that for many eyes with refractory diffuse CSDME, vitrectomy offers the potential for improved vision and reduced macular edema. Moreover, most series and anecdotal evidence suggest that the VA improvements and reductions in CSDME are long-lasting and require few retreatments.
How Vitrectomy Improves Vision
Several mechanisms have been proposed to explain the beneficial effects of vitrectomy. Separation of the posterior hyaloid relieves vitreomacular traction in both the anteroposterior and tangential dimensions by creating a PVD. The premacular cortical vitreous is known to contain contractile cells such as fibroblasts, astrocytes, and macrophages, and traction on the macula is relieved with removal of this potentially contractile tissue. Vitreoschisis—or the split hyaloid—is frequently seen in eyes with refractory CSDME. One study demonstrated that eyes with early proliferative diabetic retinopathy and macular traction have a high rate of vitreoschisis and histologic evidence of potentially contractile neovascularization into the posterior vitreous cortex.23 Others later described a similar high rate of vitreoschisis in PDR eyes with traction.24 Electron microscopy has shown multilayered sheets of potentially contractile vitreous and RPE cells in the epimacular posterior hyaloid tissue removed from CSDME eyes, and OCT studies have shown the reduction of edema following the relief of this tangentially oriented macular traction.25,26
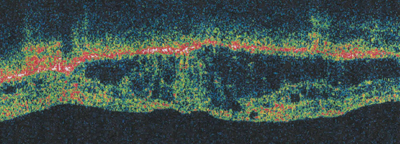 |
| Figure 3a. Pre-vitrectomy OCT in an eye without a visibly opacified posterior hyaloid membrane, VA = 20/400. |
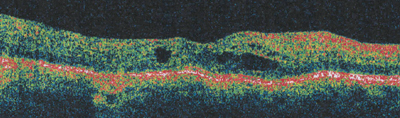 |
| Figure 3b. Three-month post-vitrectomy OCT, VA = 20/50. |
Several authors have suggested the benefits of removing the internal limiting membrane during vitrectomy; no series has shown deleterious effects. One group reported better VA and anatomic outcomes, faster CSDME resolution, and fewer recurrences with ILM peeling than all previously published series that evaluated vitrectomy without specific ILM attention.12 In other work, ILM peeling led to resolution of CSDME and VA improvement in 100 percent of eyes that failed previous vitrectomy for CSDME.27
We feel ILM peeling should be safely attempted in all cases of vitrectomy for CSDME. At a minimum, successful ILM removal insures complete removal of the posterior hyaloid from the macula—a surgical endpoint that can at times be unknowingly elusive, particularly in cases of vitreoschisis. Intrinsic pathologic effects of the ILM on the macula have also been suggested. The ILM in diabetic eyes contains increased levels of fibronectin, laminin and collagen.28,29 And peeled ILM specimens from CSDME eyes have been shown to be several times thicker than normal ILM, possibly contributing to altered fluid shifts between the vitreous and retina.30
Vitrectomy may treat CSDME by other mechanisms as well. Higher vitreous levels of VEGF, sICAM-1, IL-6 are seen in diffuse CSDME eyes than in control eyes, and their levels correlate with the severity of diabetic retinopathy, degree of angiographic fluorescence, and central foveal thickness.31,32 Removal of these permeability factors may allow reduction of CSDME. Vitrectomy has been shown to lead to an approximately tenfold increase in vitreous cavity oxygenation,33 and this may improve retinal oxygenation and perfusion, potentially contributing to decreased CSDME.
In recent years, treatment regimens for diffuse refractory CSDME have expanded, improved, and become more complex. The use of intravitreally injected triamcinolone acetonide has grown exponentially in the past five years. From the 2001 American Society of Retinal Specialists Preferences and Trend Survey, 61 percent of respondents would treat an eye with diffuse refractory CSDME, maximal macular laser, and VA of 20/100 with vitrectomy versus 13 percent that would treat this eye with an intravitreal steroid injection (Mittra RA and Pollack JS, American Society of Retina Specialists, Preferences and Trends Survey). Since then, many published and anecdotal reports have demonstrated the efficacy of intravitreal triamcinolone in the treatment of CSDME, at least in the short term, and this has led to a dramatic shift in treatment preferences.
From the 2004 PAT Survey, only 14 percent chose vitrectomy over the 81 percent that preferred intravitreal steroid use for an eye with the same refractory CSDME characteristics. Post-injection problems with intravitreal steroid use, such as elevated intraocular pressure, cataract formation, and recurrent CSDME several weeks to months after injection, however, have prevented its ubiquitous use as a permanent solution in all such eyes.
Slow-release steroid implants made with fluocinolone (Controlled Delivery Systems, Bausch & Lomb) and dexamethasone (Posurdex, Allergan) are being evaluated for the longer-term treatment of CSDME. Anti-VEGF drugs, used largely for the treatment of choroidal neovascularization, are also gaining increasing attention for the treatment of CSDME. Intravitreally-injected pegaptanib (Macugen, OSI Eyetech Pharmaceuticals) has been shown to improve or maintain VA, reduce macular volume, and reduce the need for laser compared to control/sham injections,34 though it currently does not have a label indication for this use. Bevacizumab (Avastin, Genentech) is now being intravitreally injected off-label to treat CSDME, though no data as to its widespread efficacy has been published.
The role of vitrectomy among the increasing treatment options for refractory CSDME needs definition and refinement. Vitrectomy has the potential to lead to long-lasting good outcomes for some eyes and should be in the armamentarium of all vitreoretinal surgeons. More definitive indications for vitrectomy over steroid or anti-VEGF therapy include CSDME associated with clinically and OCT-demonstrated posterior hyaloidal traction and CSDME refractory to multiple attempts at intravitreal pharmacologic treatment or CSDME in eyes with complications of pharmacologic therapy.
The results of larger multicenter, controlled, prospective, randomized trials (e.g. SCORE and DRCR.net, NEI/NIH) are needed to adequately demonstrate the efficacy of both vitrectomy and pharmacologic therapy for all types of refractory CSDME, and importantly, to compare treatment regimens against one another and evaluate the effectiveness of combination therapies. Until then, the judicious use of all available treatments allow vitreoretinal specialists to offer patients with once devastating refractory VA loss, the potential for improved vision with anticipated increasing effectiveness.
Dr. Hassan practices at Associated Retinal Consultants and William Beaumont Hospital, in Royal Oak. He is a clinical assistant professor of biomedical sciences at Oakland University, Rochester, Mich. Contact him at tsahassan@yahoo.com.
1. Early Treatment Diabetic Retinopathy Study Report Number 1: Photocoagulation for diabetic macular edema. Arch Ophthalmol 1985;103:1796-1806.
2. Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema: Long-term visual results. Ophthalmology 1991;98:1594-1602.
3. Schepens CL, Avila MP, Jalkh AE, Trempe CL. Role of the vitreous in cystoid macular edema. Surv Ophthalmol 1984;28(suppl):499-504.
4. Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology 1988;95:1335-1339.
5. Hikichi T, Fujio N, Akiba J, Azuma Y, Takahashi M, Yoshida A. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology 1997;104:473-478.
6. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes: Results of a pilot study. Arch Ophthalmol 1991;109:654-9.
7. Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology 1992;99:753-759.
8. Kaiser PK, Riemann CD, Sears JE, Lewis H. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol 2001;131:44-49.
9. Van Effenterre G, Guyot-Argenton C, Guiberteau B, Hany I, Lacotte JL. [Macular edema caused by contraction of the posterior hyaloid in diabetic retinopathy: Surgical treatment of a series of 22 cases]. J Fr Ophthalmol 1993;16:602-610.
10. Harbour JW, Smiddy WE, Flynn HW Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol 1996;121:405-413.
11. Pendergast SD, Hassan TS, Williams GA, Cox MS, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol 2000;130:178-186.
12. Gandorfer A, Messmer EM, Ulbig MW, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina 2000;20:126-133.
13. Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol 1996;122:258-260.
14. Kadonosono K, Itoh N, Ohno S. Perifoveal microcirculation before and after vitrectomy for diabetic cystoid macular edema. Am J Ophthalmol 2000;130:740-744.
15. Ikeda T, Sato K, Katano T, Hayashi Y. Vitrectomy for cystoid macular oedema with attached posterior hyaloid membrane in patients with diabetes. Br J Ophthalmol 1999;83:12-14.
16. Otani T, Kishi S. Tomographic findings of foveal hard exudates in diabetic macular edema. Am J Ophthalmol 2001;131:50-54.
17. Ikeda T, Sato K, Katano T, Hayashi Y. Attached posterior hyaloid membrane and the pathogenesis of honeycombed cystoid macular edema in patients with diabetes. Am J Ophthalmol 1999;127:478-479.
18. La Heij EC, Hendrikse F, Kessel AG, Derhaag PJ. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefe's Arch Clin Exp Ophthalmol 2001;239:264-270.
19. Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: The role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol 2001;132:369-377.
20. Otani T, Kishi S. A controlled study of vitrectomy for diabetic macular edema. Am J Ophthalmol 2002;134:214-219.
21. Talagi H, Otani A, Kiryu J, Ogura Y. New surgical approach for removing massive foveal hard exudates in diabetic macular edema. Ophthalmology 1999;106:249-256.
22. Takaya K, Suzuki Y, Mizutani H, Sakuraba T, Nakazawa M. Long-term results of vitrectomy for removal of submacular hard exudates in patients with diabetic maculopathy. Retina 2004;24:23-29.
23. Faulborn J, Bowald S. Microproliferations in proliferative diabetic retinopathy and their relationship to the vitreous: Corresponding light and electron microscopic studies. Graefe's Arch Clin Exp Ophthalmol 1985;223:130-138.
24. Schwartz SD, Alexander R, Hiscott P, Gregor ZJ. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology 1996;103:323-328.
25. Jumper JM, Embabi SN, Toth CA, McCuen BW, Hatchell DL. Electron immunocytochemical analysis of posterior hyaloid associated with diabetic macular edema. Retina 2000;20:63-68.
26. Gandorfer A, Rohleder M, Grosselfinger S, Haritoglou C, et al. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol 2005;139:638-652.
27. Kimura T, Kiryu J, Nishiwaki H, Oh H, et al. Efficacy of surgical removal of the internal limiting membrane in diabetic cystoid macular edema. Retina 2005;25: 454-61.
28. Kohno T, Sorgente N, Doodnight R, Ryan SJ. Alterations in the distribution of fibronectin and laminin in the diabetic human eye. Invest Ophthalmol Vis Sci 1987;28:515-521.
29. Ljubimov AV, Burgeson RE, Butkowski RJ, Couchman JR, et al. Basement membrane abnormalities in human eyes with diabetic retinopathy. J Histochem Cytochem 1996;44:1469-1479.
30. Matsunaga N, Ozeki H, Hirabayashi Y, Shimada S, Ogura Y. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina 2005;25:311-316.
31. Funatsu H, Yamashita H, Sakata K, Noma H, et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 2005;112:806-816.
32. Funatsu H, Yamashita H, Ikeda T, Mimura T, et al. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology 2003;110:1690-1696.
33. Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am J Ophthalmol 2005;139:302-310.
34. Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, et al.; Macugen Diabetic Retinopathy Study Group. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747-57.



