Protocol T’s Algorithm
Though physicians will develop their own algorithm for treating DME, the process used in Protocol T can serve as a well-studied starting point.
Jack Wells, MD, a retinal specialist in Columbia, S.C., and lead author on the Protocol T study, says the study’s algorithm is sometimes unfortunately viewed as overly complicated. “Many physicians—with some justification—claim it’s complicated,” he says. “It’s really not that complicated. Basically, you start injecting with intravitreal anti-VEGF and keep injecting for six months until you get resolution. After six months, if there’s persistent edema, you perform laser, but you don’t keep giving injections after six months if it’s not improving. If, however, there’s been gradual improvement at six months, you don’t do the laser but instead keep giving the injections. I think the algorithm is hard for people in clinical practice to follow because they can get impatient. After two, three or four injections, if the patient doesn’t show dramatic improvement, some might say the treatment isn’t working and switch to another anti-VEGF drug or to steroids. But this algorithm demands patience, that you keep plugging away until, ultimately, you get to a good place. It’s a gradual, continuous improvement over a span of months. It does require a lot of treatment.”
The First Line
Physicians reference the results of Protocol T when describing their first-line options, since the drugs seemed to perform differently for different patient presentations.
Dr. Wells says the Protocol T investigators prespecified an analysis based on baseline vision, hypothesizing that eyes with worse vision might have thicker maculas because they had higher vascular endothelial growth factor levels. “A drug that has a higher VEGF binding capacity might give a better outcome,” explains Dr. Wells. “So, we drew the acuity line at 20/50, because that was the median visual acuity in all the previous DRCR studies of DME. In the first year, we found that in eyes with better than 20/50 vision at baseline there was no difference in visual improvement between the three drugs; all three gained a mean of about eight letters. However, in eyes with vision worse than 20/50, aflibercept was better than the other two at one year: Eylea eyes gained 19 letters; Lucentis eyes gained 14; and Avastin subjects gained 12, differences that were highly statistically significant. The other thing we found at the first year follow-up was that eyes treated with Avastin didn’t show as much of a reduction in edema as eyes treated with the other two drugs, regardless of baseline vision. So, Avastin didn’t give the same drying effect. This didn’t seem to matter in terms of vision in the better baseline vision group, but it did seem to matter in the group with worse baseline vision.
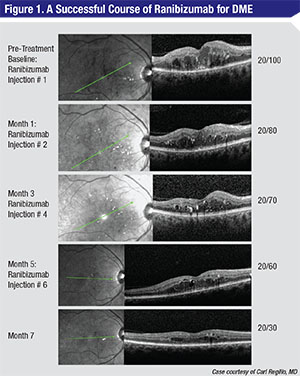 |
| This 58-year-old diabetic macular edema patient was treated with monthly injections of ranibizumab (Lucentis) for seven months (not all follow-up visits are shown). The patient shows improvement each month, and his vision improved from 20/100 to 20/30. |
In the second year, we found that the vision gains and OCT improvement that were seen at one year were sustained,” Dr. Wells adds. “So, you still had a very good improvement in vision and a reduction in DME, and that was maintained with a little more than half as many injections and with much less laser than was given in the first year: In the first year, eyes got nine or 10 injections, and in the second year they got five or six. Statistically, the difference between Eylea and Lucentis that was seen in year one diminished. By year two, the Eylea group had gained a mean of 18 letters, and Lucentis had gained a mean of 16, a difference that was no longer statistically significant. The difference between Eylea and Avastin was still statistically significant, however. A lot of people look at these results, then, and say, ‘It doesn’t matter which drug you start with.’ There’s some truth to that, because eventually you’re going to get to the same place, but in that first year Eylea is so much better that I wouldn’t want to deny patients that rapid improvement over that first year. Also, there were half as many laser treatments administered in the second year as in the first.”
Following the results of the study, Charlotte, N.C., retinal specialist Andrew Antoszyk alters his approach based on the patient’s baseline vision. “Based on the results of Protocol T, if the patient has good visual acuity (20/32 to 20/40), I’ll begin treatment using Lucentis because of its better drying effects than Avastin and the availability of pharmaceutical-funded co-pay assistance,” he says. “If they have worse VA (20/50 or less), then I’ll start with Eylea because of the better visual acuity results in the first year of therapy. In situations where there are insurance issues for coverage of the injections, I’d use Avastin. The availability of drug makers’ assistance programs makes the patient’s out-of-pocket cost for these injections less.”
Some clinicians wonder if the presence of macular non-perfusion will alter therapy. “If the macula is still swollen, I’ll still try to treat it,” says Carl Regillo, MD, director of the retina service at Wills Eye Hospital. “I’ve had scenarios where there’s obvious non-perfusion and you wouldn’t think there’d be a visual acuity improvement—but there still can be if there’s also edema. There are also scenarios where non-perfusion can improve on anti-VEGF therapy. So, if there’s edema, perfused or even non-perfused, I’ll start treatment and try to make the edema, and the vision, as good as possible. The presence of macular non-perfusion doesn’t influence things at first—it explains things. It could influence whether I stop treatment earlier, and the intensity of the treatment may vary in the patient with non-perfusion.”
Gauging Improvement
Just as they use Protocol T as a jumping-off point for choosing their initial therapy, physicians also use its protocol as a guide for determining the need for re-injection.
Dr. Wells says the study used as parameters a five-letter change in vision, better or worse; and a 10-percent change on OCT, for better or worse. “Basically, after you started treatment, if the patient came back for his next visit and the vision was five letters better or worse and/or the OCT was 10 percent better or worse, you retreated,” he explains. “It was only if there was no change for two consecutive visits per these definitions that treatment was withheld. Injections then only resumed if the patient got worse. Then, at six months, if there was improvement compared to the previous visits, you didn’t do laser, but instead kept injecting. So, after six months, you would only add laser if there was no improvement or the patient was getting worse.”
Dr. Antoszyk modifies Protocol T’s algorithm slightly. “If the patient has had a five-letter decline from his last visit or an increase in the central sub-field thickness of 10 percent on OCT, then I’ll retreat,” he says. “Part of this decision is determined by symptoms. If the patient isn’t bothered by this 10-percent change on OCT or the change in vision, I’ll discuss the possibility of waiting a month to see what the disease does at that point. During the discussion, I’ll show him the images and discuss the potential result of having edema over an extended period of time that can lead to moderate vision loss.
“If, after the treatments, the individual returns with excellent vision—which I’d define as 20/20, and the OCT is in the normal range—somewhere less than 300 µm—then I’d hold treatment and have him come back in a month,” Dr. Antoszyk adds. “If the condition is the same then, I’d hold and have the patient come back in two months. If, after two months, he is still stable, in other words there’s no change in visual acuity or recurrent fluid formation, then I’d go to four months. The visits would then be every four months for the first year. If there’s no recurrence of macular edema, then follow-up would be dictated by the severity of the retinopathy. In other words, if it’s mild, then follow-up could be yearly. If it’s moderate, the follow-up could be every six months, and if it’s severe, every four months.”
Dr. Regillo says he tends to take more of a traditional PRN approach, which he describes as a little bit of a modified treat-and-extend. “Because many patients with DME can come off treatment at some point in their course—maybe six to 12 months into it—and because most need frequent, regular injections to get to their best point, which could be six to 12 months of therapy, for me treatment is essentially monthly until the macula is dry for all significant, center-involving DME, regardless of the drug used,” he explains. “And the term ‘significant’ means, for a given patient, enough edema to start to cause some decreased acuity. Obviously, this doesn’t mean treating every little bit of edema. Some patients will do well on their own with just small amounts of edema, even center-involving edema, and have good vision. I’ve always favored the on-label drugs, and the more edema there is, the more likely I am to use Eylea. Nothing’s hard and fast though—we have to take all the information and put it together for any one patient.
“I’ll treat until the macula gets to be as good as I think it’s going to get,” Dr. Regillo continues. “That may mean it’s not completely dry, but I’ll keep pushing until the macula looks like it’s not getting any better. If it’s dry, then great; that might be 60 or 70 percent of patients. And if it’s not quite dry but there’s good vision, I might be happy with that.”
When to Switch
Unfortunately, not every patient will respond to the initial anti-VEGF therapy, and surgeons say there are no good, well-controlled studies looking at the various effects of switching to an alternative anti-VEGF drug or to a steroid—physicians just have to rely on their clinical experience and what they know about different drugs’ mechanisms.
Dr. Antoszyk likes to give the anti-VEGF therapy some time before he’ll contemplate a switch. “Usually, I’ll treat a patient with an anti-VEGF drug for six months before I consider switching to an alternate therapy,” he says. “So, if I start with Lucentis or Avastin and, after six months, the patient has come to a standstill in response, I’d consider switching to Eylea to see if there might be an additional benefit. This is a good way to see if you’ll get additional benefit without the side-effects of intraocular steroids, which include ocular hypertension and cataract formation. However, if the patient doesn’t get a response after six Eylea injections, I’ll switch to an Ozurdex implant. If they get a good response from Ozurdex, but require it on a repeated basis, they would be a good candidate for an Iluvien implant.”
Dr. Wells, however, thinks some patience is in order when it comes to treating DME, and that it can take some time for the drugs to get the eye to an optimal condition. “Since I was in the study, I understand that it requires a lot of treatment to get to a good place, so I keep plugging away,” he says. “We at the DRCR.net have even polled physicians about how many anti-VEGF injections they’ll give before switching to a steroid, and they usually reply that it’s between three and six. However, if you look at the study, nine to 10 injections were given in the first year, so most got an injection almost every time they came in. If you keep plugging away with the DRCR algorithm, you eventually get there. I’ll start with Lucentis in eyes with good vision because I like to see the edema go away, and I don’t think I’m going to see that as much with Avastin. However, if there’s an insurance coverage issue for the patient, I’ll start with Avastin. If I don’t get the response I want from Lucentis treatment, I’ll consider switching the patient to Eylea, which dries the edema a little bit better, especially in the worse-vision eyes.
“I stick to anti-VEGF therapy for a year or even more as long as there’s improvement. It’s really only after a year that I’ll think about doing something else,” Dr. Wells continues. “In my clinic, I add laser treatment if they’re not getting better. In some cases, though, you perform an angiogram and observe this massive cystoid edema. I view that more like an inflammatory situation, and will therefore think of using a steroid at an earlier stage, such as six months, if they haven’t gotten better.”
As Dr. Wells alluded to, physicians still use laser in select cases as an adjunctive therapy. “It’s still an effective technique,” Dr. Regillo avers. “However, I treat with laser much less often now, and do so less aggressively. I’ll use laser for non-center involving edema, especially with angiographically identifiable leaking microaneurysms that I can target with focal laser. I’ll occasionally introduce it later in the course of anti-VEGF therapy if I’ve been able to dry things up but I get some recurrences of the edema, especially if it’s non-center involving. When administering the treatment, I don’t tend to push it quite as close to the center of the macula as we would have done in the old days when laser was all we had. Now it’s careful and cautious use of laser, introduced for very specific, non-center-involving scenarios.”
Safety Signals
Over the years, some physicians have wondered about the systemic impact of frequent injections of an anti-VEGF drug, especially in patients who already have health problems, as diabetic patients often do. Though retinal specialists say there’s been nothing overtly negative reported on the topic of safety of anti-VEGF drugs, you can adjust your approach in certain patients.
Dr. Antoszyk says the timing of a patient’s health events might change therapy. “None of the currently published studies have been powered adequately enough to do an overall clinical safety assessment,” he says. “However, with the anti-VEGF drugs, there have been signals that there may be an increased rate of [Anti-platelet Trialists’ Collaboration] events. In my practice, if a patient has had a stroke or heart attack within the past month, I might defer treatment—especially in DME—and may consider switching to a steroid within the first three months of an event. One of the issues in these patients is, once they’ve had an APTC event, they’re at an increased risk for another, so is it the anti-VEGF that’s causing the next event or their natural history due to multiple risk factors? It is possible that future meta-analyses may provide the answer to this question.”
Dr. Regillo will also keep systemic risks in the back of his mind when initiating treatment. “Diabetics in general, at a given age, tend to be sicker from a cardiovascular standpoint, and probably are at an increased risk across the board for cardiovascular or cerebrovascular events,” he says. “So, theoretically, these drugs might increase the risk, though there’s no proof to this day that they do. It doesn’t alter what I do. I might speak to someone with a history of stroke about this theoretical risk, but it’s unlikely to alter what I do.
“If someone had a recent stroke and has a lot of other medical issues, I might offer a steroid,” Dr. Regillo continues. “Theoretically, we might think of one anti-VEGF agent as being safer than another—the pharmacokinetics seem to suggest that Lucentis would be the safest from a systemic standpoint, or have the least exposure to risk. But studies really haven’t borne out any differences between the drugs. If anything, they’ve been conflicting. It’s hard to say if there really are any safety differences at this time.” REVIEW
Dr. Regillo has consulted for Genentech and Regeneron. Dr. Wells has received grant support from Genentech, Regeneron and Allergan. Dr. Antoszyk has no financial interest in the products discussed.
1. Wells J, Glassman A, Ayala A, The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. N Engl J Med 2015;26;372:13:1193.



