It's always difficult to improve on a clever, effective procedure such as Descemet's stripping automated endothelial keratoplasty, but surgeons and companies may have been able to do it by way of endothelial inserters that do away with a lot of the trauma involved with grasping and folding delicate grafts with forceps. Here's a look at the DSAEK insertion instruments that are either available in the
Angiotech's Tan EndoGlide
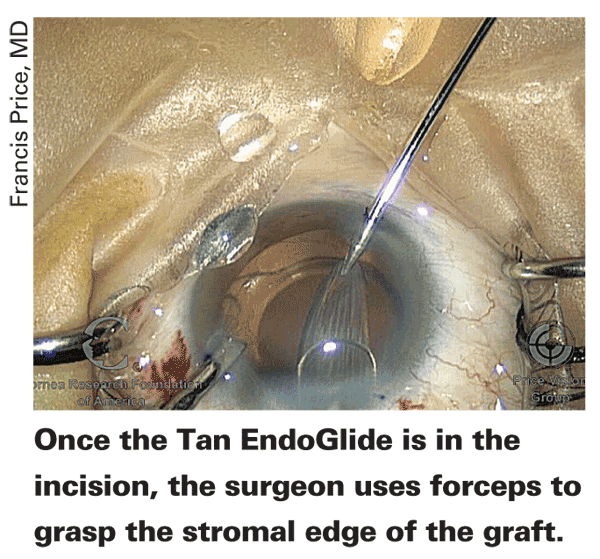
The EndoGlide was launched in the fall of 2009, and it consists of a base, a cartridge and an introducer. To use it, the surgeon places the donor graft in a small depression in the base, endothelium-side up, and applies a small amount of dispersive viscoelastic to the endothelial surface in a thin strip. The surgeon positions the cartridge alongside the graft in the base and injects some BSS into it for lubrication. The surgeon puts forceps through the cartridge, coming out alongside the graft. He then grasps the stromal edge of the graft and pulls it into the cartridge. Due to the internal design of the cartridge, the act of pulling the graft into it causes the tissue to coil into a double coil, with no endothelial surfaces touching. The physician then attaches the EndoGlide introducer to the cartridge at the rear of the device, sealing it shut on that side. It's now a fully assembled EndoGlide, and can be removed from the base and rotated right side up.
The EndoGlide goes through a scleral tunnel incision approximately 4.5 mm in width. A chamber maintainer and a paracentesis for forceps are also necessary. Once the glide is inserted through the incision, the surgeon uses a set of forceps to reach across the chamber from the other side and pull the graft out of the inserter by grasping the stromal edge.
Wilmer Eye Institute's Albert Jun, MD, has worked with the EndoGlide, and says he thinks it's got a couple of helpful features. "It's relatively compact, and allows the tissue to scroll as it's loaded," he says. "Also, it can be inserted completely into the wound, sealing it. Once you get into the eye, the anterior chamber is stable and deep." For the forceps to be used inside the eye, Dr. Jun prefers a disposable vitrectomy forceps that he can bend to his liking.
Boston
Dr. Jun says that, once the glide is in the eye, grasping the edge of the graft with the forceps can be tricky if you leave it completely inside the barrel of the inserter. "It then becomes difficult to retrieve because you can't really see things too well at that point," he explains. "The cornea is usually cloudy, and the material of the inserter is a translucent, but not transparent, plastic. Because of that, I leave 0.5 or 1 mm of folded tissue just extending past the edge of the barrel to grab onto with forceps."
Though Dr. Jun hasn't studied endothelial cell counts specifically in relation to the use of the instrument, he says the "clarity of grafts on day one is clearly different from what you see with folding forceps, either due to decreased trauma, or perhaps because the endothelium recovers more quickly." He says his graft dislocation rate was low before he began using the EndoGlide, and it's remained so. "I don't get the impression that it's different from my previous rate of around 5 percent," he says.
Dr. Jun is, however, wary of eyes that have iris issues. "If there's any trauma or previous changes to the iris, where the iris isn't robust, the glide can be a little difficult to maneuver into the anterior chamber," he says. "In my practice, I have referral patients who have had multiple other surgeries, possibly with lenses in the sulcus, irises with large defects and perhaps atrophy. I have to maneuver the leading edge ramp of the instrument over these structures, otherwise they'll catch it and drag it across, which you don't want to happen.
"And the approaches are important," Dr. Jun continues. "You need to have a main wound and an accessory wound across from it in order to reach in and grab the graft, so it can be challenging if it's a deep-set eye or orbit or a high brow or high nasal bridge. In those situations, it can be kind of challenging to get the instruments around the eye at the correct angles. It works well for patients with good orbital access."
Dr. Rapoza feels the step of loading the donor tissue into the EndoGlide
is crucial in obtaining good results. "The fashion in which you pull the tissue into the inserter is the most important surgical step of using the EndoGlide," he says. "I carefully grasp the leading edge of the donor lamellae bisecting the graft then draw the tissue into the cartridge using viscoelastic to coat the endothelium and the viscoelastic cannula on the edge of the tissue to guide the insertion. If you don't do a good job and the tissue is not symmetrically rolled up, additional manipulation of the graft in the eye will be required with the potential for further damage to the endothelium." He says the main issue he's run into with the instrument is difficulty getting the pull-through forceps to disengage from the tissue. It's happened to him twice. "You're opening the jaws some but it doesn't let go," he says. "In those cases, I try to keep it open and move the forceps toward the brow and then the mouth, swinging it to try to loosen any adhesion. In one case, I had to put a second instrument into the eye, like a y-hook, to push the tissue off of the platform."
Keramed's EndoInjector
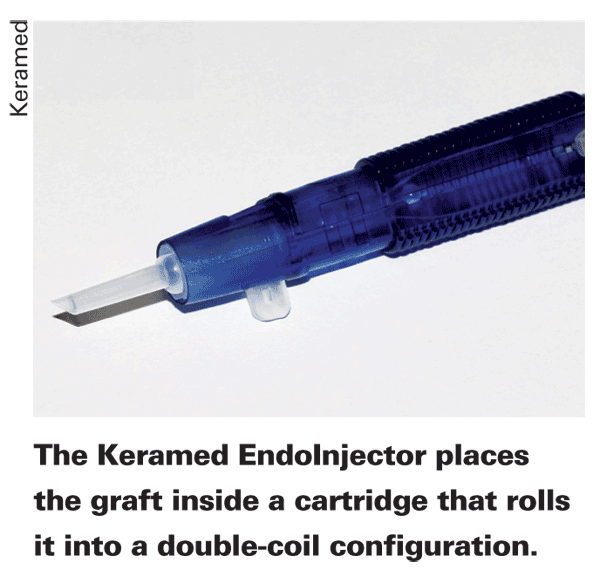
Previously called the EndoShield, the device's developer, Keramed President Yichieh Shiuey, MD, says the device functions closer to an intraocular lens injector than to a tissue inserter. "With other devices, you may need two hands, which can be tricky, and also you may have to take forceps and pull the graft through the lumen of the device," says Dr. Shiuey. "Or another device may use a platform that has to be rolled in and out of a tube, with the issue of separation of the graft from the platform. In some cases, the surgeon may have to shake the platform inside the anterior chamber to get the graft off or use a hook to take it off the platform, which can get complicated. With the EndoInjector, there's a cartridge with proprietary shape on the inside where there's a central ridge that causes the graft to form a double coil. A double coil allows the injector to go through a much smaller incision. Theoretically, an ideal double coil inserter could get a graft through a sub-3 mm incision. Even a single coil, where there's no overlap of the graft, would have a lower limit of 4 mm. But in the real world, for a double coil, we recommend a 3.2-mm incision, while for a single coil inserter it's about 4.5 mm for practical purposes to get the barrel of the inserter into the anterior chamber. For a surgeon's very first case, a 3.5-mm incision probably would be best."
To use the EndoInjector, the surgeon puts the graft endothelium-side up into the cartridge, with the stromal side against the cartridge lumen, and closes it for loading. "Once you put the graft in the cartridge and close the wings, the graft is set in a position where the sides are starting to curl in," explains Dr. Shiuey. "The edges of the graft are curled in a little, and the barrel of the lumen narrows as you go toward the tip, so by the time the graft reaches the end of the cartridge, the edges almost touch endothelium, but don't actually touch." The surgeon doesn't have to insert the entire cartridge into the anterior chamber, just get the bezel past the entry wound. He can then get the graft to unfold in the anterior chamber by pushing on a sliding thumbrest.
In terms of results, Dr. Shiuey says he's currently getting data from users showing an 18 percent endothelial cell loss at six months post-transplant. "This is favorable and appears to be stable over time," he adds. There have been 100 endothelial insertions with the device so far, and there haven't been any cases of graft failures or slipped grafts.
The EndoInjector is currently working its way through the U.S. Food and Drug Administration 510(k) process. Dr. Shiuey is hoping to have approval for the device within four to six months.
Fischer Surgical's NCI
When
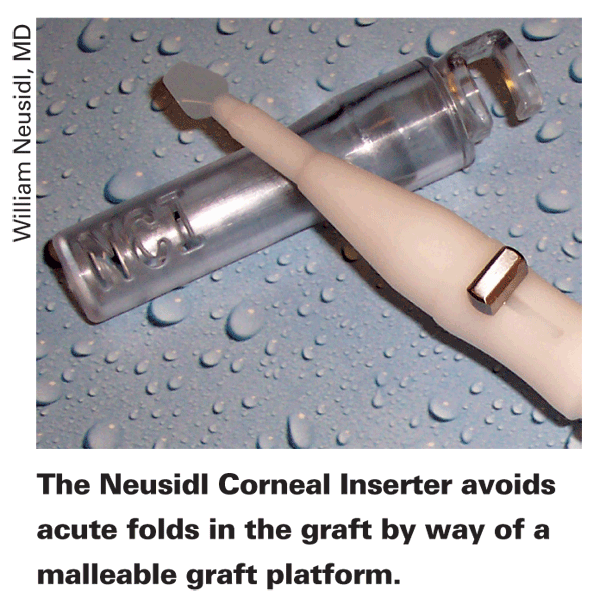
With the NCI, the surgeon places the graft stromal side down on an extended platform that is shaped similar to a small, flat spoon with a slight curve on the edges. The platform is composed of a thin, malleable plastic that rolls up readily when retracted into the elliptical barrel of the NCI. The internal diameter of the elliptical tube is engineered so that the most common graft sizes used for EK (8 to 8.5 mm), could be rolled into an elliptical configuration with no folds and no overlap of tissue. "Depending on the thickness of the graft, there may be fine creases but there are no folds in the graft when rolled up into the protective elliptical chamber of the NCI," says Dr. Neusidl. "Vital dye studies have shown that a taco or tri-fold produces linear cell damage in areas where you see an acute fold."
The NCI has incorporated irrigation into the hand piece. The irrigation is compatible to the standard irrigation set-ups used for cataract surgery. The irrigation
keeps the anterior chamber formed during delivery of the donor graft and also facilitates the release of the tissue off of the platform. This eliminates the need for a separate anterior chamber maintainer.Wills Eye Institute corneal specialist Sadeer Hannush has implanted 85 NCI grafts, and feels it's an improvement over a forceps technique. "First, the actual tissue is never pushed through a tunnel," avers Dr. Hannush. "With the NCI, there's no translational movement, meaning it's just rolled and retracted. The whole platform is retracted, while the tissue stays stationary on it. Second, the tissue isn't folded. Instead, it's kind of rolled, with no edge overlap and no creases or endothelium-to-endothelium touch."
The incision diameter necessary to use the NCI is 5.5 mm, a little wider than that used for forceps. It can be used with either a scleral tunnel or clear corneal incision. "There should always be a paracentesis in case you need a second instrument to free up the tissue from the platform," notes Dr. Hannush. "Many surgeons have complained that, because the tissue adheres so well to the platform that when you get it into the anterior chamber it doesn't release. However, if you're patient and allow the irrigating flow to go into the anterior chamber, sometimes a few maneuvers are all that are required to release it."
Portland
In an analysis Dr. Hannush presented at the April meeting of the American Society of Cataract and Refractive Surgery, he looked at 35 NCI cases and 248 cases done with folding forceps. At three months, the mean cell count for all the NCI cases was 1,691 cells/mm2. The mean for forceps at that time point was 1,499 cells/mm2, a difference that was statistically significant (p=0.04). At six months, 25 NCI cases were available for analysis, vs. 223 forceps cases. For the NCI, at six months the mean cell count was 1,640 cells/mm2, compared to 1,431 cells/mm2 for folding, which was also statistically significant (p=0.048). In terms of complications, Dr Neusidl presented preliminary findings at ASCRS from surgeons who have been using the NCI. At that time there were 218 cases using the NCI with no reported primary graft failures and five cases of graft dislocations (2.3 percent).
An important pearl for using the instrument, says Dr. Hannush, is that both the tissue and the platform need to be dry when the donor graft is placed on the platform. To this end, he wicks any wetness that's around the graft after it comes off the punch block. "It's OK to connect the irrigation to the back of the NCI, but just don't start the flow, yet," he advises. "I prefer to load the tissue and retract it before I connect the irrigation, so there's no possibility that a few drops of irrigant will wet the platform. I suggest starting with a 40 cm bottle height.
"Also, you don't want to put the NCI into the anterior chamber until you've pushed out the one remaining air bubble," Dr. Hannush continues. "You want the bubble out of the lumen of the NCI before you enter the eye, because you don't want a bubble in front of the graft when you're in the eye."
Dr. Hannush says he's been very pleased with the NCI's results in his patients. "Almost all of them have a very rapid improvement in uncorrected vision after surgery," he says.
Ocular Systems Inc.'s Endosaver
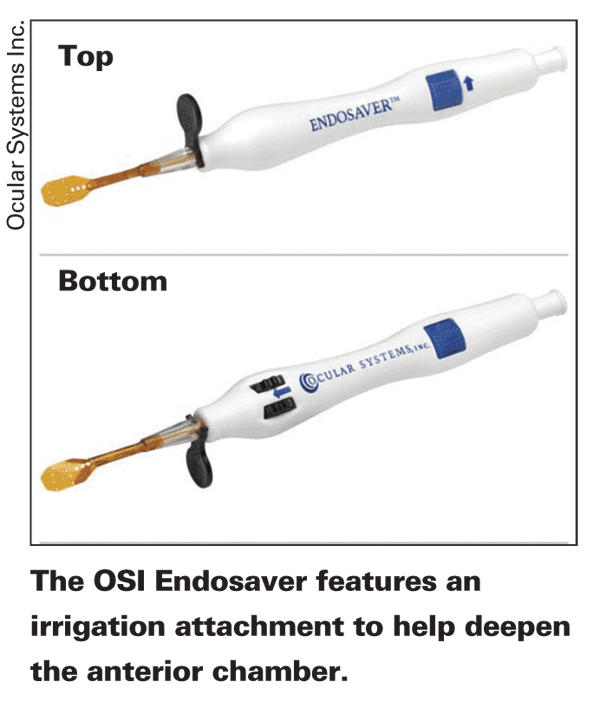
The Endosaver consists of a handle and an internal mechanism for rolling the graft and retracting it into the body of the instrument for delivery into the eye. It can handle grafts up to 8.5 mm in diameter and up to 175 µm thick, and it needs a 4-mm incision or larger for entry into the anterior chamber. It also has an irrigation attachment.
To use it, the surgeon places the graft onto the platform, called the carrier, endothelium-side up, taking care not to let any part of the graft dangle off the front end. He then turns a thumbscrew, which brings the carrier and tissue back into an insertion sheath. As it comes back, the carrier rolls and the tissue rolls with it, until it's small enough to fit in the sheath. The surgeon then turns the device upside down, holding small deployment wheels situated on the body of the device perfectly still, so as not to damage the graft when the sheath is inserted into the anterior chamber. Once inserted, the irrigation can help deepen the chamber, and the surgeon rolls the deployment wheels. This rolling of the wheels uncovers the graft inside the eye as the sheath retracts away from it. The graft is then ready to be attached, and the device can be removed from the eye.
Duke
Heather Skeens, MD, assistant professor of ophthalmology at the Storm Eye Institute at the Medical University of South Carolina, has used the Endosaver, and feels it's less traumatic on endothelial cells.
"In the cell counts we have, I'm losing very few cells," she avers. "I like that the device also accommodates a range of graft sizes, and I've done 7 mm grafts up to 8.5 mm."
Neil Griffin, a corneal specialist in
"Once the sheath is in the eye, you can sometimes assist the unfolding process by rocking it one way or the other," Dr. Griffin continues. "If you have a small lip that hasn't unfolded, by deepening the chamber it will finish unfolding;
you need enough depth in the anterior chamber."
For corneal transplant surgeons who were already pleased with the results of DSEK and DSAEK, the introduction of inserters that have the potential to make the procedures even safer has given them even more reason to be excited about the future of corneal grafts.



