Richard Abelson, MS, Daniel Dewey-Mattia
The perceived efficacy of a drug in clinical trials and in practice may be skewed by several factors unrelated to the drug's pharmacology. The widely recognized phenomena known as the "placebo effect," as well as the "
The Placebo
The term placebo was first used medically in the 18th century to describe remedies prescribed to placate the patient rather than cure an actual disease. In modern medicine, a placebo is defined as an agent composed of physiologically inert/inactive material whose appearance is matched to the active agent in order to mask patients and/or investigators to the identity of the product they are using. Placebos in clinical research are the primary basis of comparison in determining the efficacy and safety of a pharmacological therapy; if a drug benefits the patient beyond a pre-defined threshold as compared to placebo, it's considered effective.
The first placebo-controlled clinical trial took place in 1931, testing the effectiveness of gold therapy for the treatment of tuberculosis. However, the placebo-controlled trial design didn't gain instant widespread approval, and placebo-controlled trials were rare prior to World War II. It wasn't until 1962 and the introduction of the Kefauver-Harris Amendment to the Food, Drugs and Cosmetics Act that the U.S. Food and Drug Administration required that new drug applications not only demonstrate the safety of a product, but also its efficacy. Further promoting the use of placebo-controlled studies, the FDA introduced guidelines in 1970 for the conduct of clinical trials and the determination that commercial success alone does not constitute substantial evidence of safety and efficacy.
Prior to the widespread use of placebos in trials, investigators would compare one treatment to another or to no treatment, largely due to the notion that deceiving ill patients into believing they are receiving active treatment was unethical. This issue has been partially resolved with the development of informed consent in which trial participants are notified that they may be receiving an inactive treatment.
Historical Revelations
Medicine prior to the establishment of the scientific method often included the administration of exotic cure-all therapies ranging from powdered Egyptian mummy to the gallstone of a goat, treatments now known to be useless. Many have gone so far as to refer to the history of medical treatment prior to the 20th century as a "history of the placebo phenomenon,"1,2 based on the realization that the bulk of the popularized medicinal treatments throughout history have since been proven pharmacologically ineffective. However, the ability of many of these now-disgraced agents to inspire confidence in their recipients and their strong support from respected physicians hints of the influence placebo therapy can have over the clinical signs and symptoms of disease.
The power of the placebo began to gain widespread appreciation following the research of Henry Beecher. During his deployment as an anesthesiologist for the allies in World War II, Dr. Beecher, like many other doctors, was faced with a shortage of morphine when dealing with large volumes of casualties. In one particular instance, with a patient in excruciating pain for which he was demanding alleviation prior to surgery, a member of the nursing staff attempted to appease him by giving him an injection of saline. To the astonishment of Dr. Beecher, the patient showed little sign of pain or shock throughout the ensuing surgery. In subsequent morphine shortages, Dr. Beecher repeated this placebo treatment, reporting a high rate of positive response.3
After the war, Dr. Beecher and his colleagues began to investigate the powers of the placebo. In a seminal review of 14 experiments and a total of 1,082 patients examining the effects of placebo in conditions ranging from severe postoperative pain to the common cold, Dr. Beecher found an average success rate of 35 percent.4 All but one of these studies, however, didn't contain a control group (in which patients received no treatment) and the one study for which a control was used found no difference between the placebo and no-treatment groups. Thus, the resolution of the disease seen with placebo may have been caused by a natural alleviation of symptoms with time. Nevertheless, Dr. Beecher's research did bring considerable attention to the subject of placebos, helped convince researchers that the use of placebos was both necessary and ethical and paved the way for more controlled studies of their effects.
The Placebo Effect
Clinical studies are often designed with the intent of comparing drug effects on signs and symptoms in a treatment arm to a placebo group, which ideally will portray the natural course of the disease. However, between-group comparison of results is often distorted by what is known as the placebo effect: a measurable, observable, or perceived improvement not attributable to an active treatment.
Subjective assessments, being associated with psychological variables, are generally considered more prone to the placebo effect than objective assessments. This is reflected by the exceptionally high positive response rate to placebo seen in studies of pain and psychiatric disorders, but similar effects are often seen in any study in which self-report measures are used. For example, in a study of oral antioxidant therapy for dry eye, subjective parameters were significantly improved for both active and placebo treatment groups after one month of therapy. In contrast, only antioxidant-treated patients showed significant improvements in tear thinning time, goblet cell density and metaplasia.5
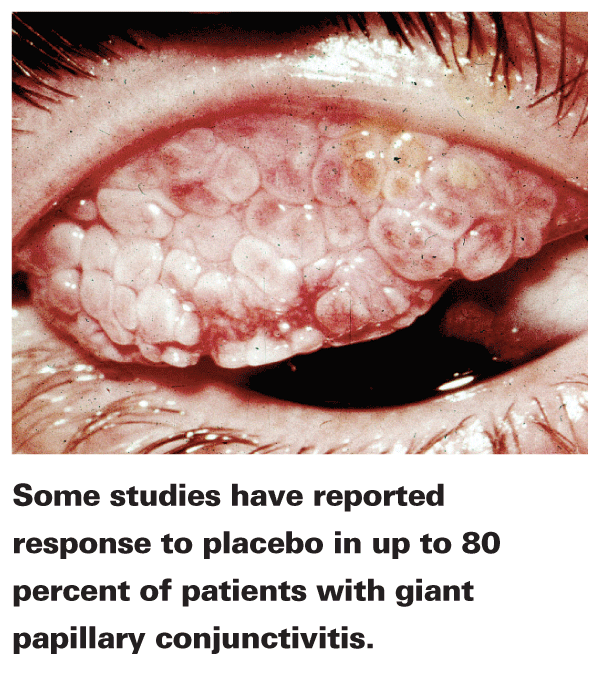
The placebo effect also manifests in non-psychological parameters. Ophthalmic solutions used as vehicle comparators in allergic conjunctivitis trials can alleviate symptoms through allergen washout. The pathophysiology of allergy requires contact time with the conjunctiva, so an artificial tear (not a true placebo, but loosely considered as such by convention) instilled in the eye can interrupt this process by washing away allergen and leading to a high frequency of positive placebo responses. For example, in a study evaluating the efficacy of azelastine 0.05% or 0.025% eyedrops for the treatment of allergic conjunctivitis or rhinoconjunctivitis, 56 percent of subjects in the placebo group had improvements in itching, lacrimation and redness.6 Likewise, others have reported placebo response rates as high as 70 percent for seasonal allergic conjunctivitis7 and as high as 80 percent for giant papillary conjunctivitis.8
Similar findings have been reported in dry-eye research. Tear substitutes used as a placebo in dry-eye trials have also shown significant improvement in tear-film stability when compared to baseline measurements. (Vehicle comparators in dry-eye trials can also cause a negative placebo effect due to irritation associated with the inactive ingredients and preservatives.)
In some circumstances, placebos may provide a greater benefit in environmental situations that are less challenging to the patient. Therefore, the use of a standardized controlled testing environment (i.e. Controlled Adverse Environment in dry eye) can help reduce the therapeutic benefit of a placebo. Also, the use of a crossover design is hypothesized to aid in managing the placebo effect by reducing the expectancy of efficacy of placebo treatment when active treatment is given first.9
Despite broad support for the existence of a placebo effect, the topic remains controversial and some researchers have called into question the magnitude and even the validity of the phenomenon. In a meta-analysis published in the New England Journal of Medicine,
Other Confounders
In addition to the placebo effect, there are other phenomena that can yield misleading results:
• The
With regard to clinical studies, the
• Regression toward the mean. The concept of regression toward the mean is based on the realization that patients are most likely to seek treatment, or enter a clinical study, for a condition when their symptoms are at their worst. This occurs most commonly in diseases with wide fluctuations in severity.
When inclusion criteria for a study require a baseline value above an individual's mean value for his condition as a whole, he may still be included in the study either by a chance high reading at a screening visit or through multiple screening attempts. As a result, the reading for his condition could appear to have improved (decreasing toward their average value) at subsequent visits, independent of whether or not he is receiving active treatment.
One method to reduce the effects of regression toward the mean is to have subjects meet inclusion criteria at more than one visit or during a run-in period, lowering the proportion of patients with baseline values outside their normal range,
• Exuberance. Patient exuberance to participate in a clinical study and meet inclusion criteria can also influence efficacy results through the inflation of self-reported outcomes during screening and baseline evaluations. Likewise, the desire of the investigator to enroll higher numbers of patients could cause him to stretch baseline scores, which may then cause a patient's condition to appear to have improved as the study progresses.
With the right design, statistical methodology that accommodates for the inherent bias, and the use of repeated measures and multivariate analysis techniques, a better estimate of the true drug effect can be achieved.
Clinicians should be aware of how the placebo effect and other potential confounders can influence both objective and subjective measurements, especially if the clinicians want to conduct a study evaluating a particular therapy in their practice. These factors should always be considered and accounted for when prescribing medications based on clinical data and when assessing patients' improvement on medication.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Margo CE. The placebo effect. Survey of Ophthalmology 1999;44:1:31-44.
2. Shapiro A. A contribution to a history of the placebo effect. Behavioral Science 1960;5:2:109-35.
3. Evans D. Placebo: Mind over matter in modern medicine.
4.
5. Blades KJ, Patel S, Aidoo KE. Oral antioxidant therapy for marginal dry eye. Eur J Clin Nutr 2001;55:7:589-97.
6. Giede-Tuch C, Westhoff M, Zarth A. Azelastine eye-drops in seasonal allergic conjunctivitis or rhinoconjunctivitis - A double-blind, randomized, placebo-controlled study. Allergy 1998;53:9:857-62.
7. Leino M, Ennevaara K, Latvala AL, et al. Double-blind group comparative study of 2% nedocromil sodium eye drops with 2% sodium cromoglycate and placebo eye drops in the treatment of seasonal allergic conjunctivitis. Clin Exp Allergy 1992;22:10:929-32.
8. Friedlaender MH, Howes J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group
9. Eccles R. Importance of Placebo Effect in Cough Clinical Trials. Lung;188:S53-S61.
10. Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 2001;344:21:1594-602.
11. McCarney R, Warner J, Iliffe S, et al. The