In 1907, Ludwig Halberstaedter and Stanislaus von Prowazek discovered Chlamydia trachomatis within conjunctival scrapings of trachoma patients. The two scientists named the unusual inclusion bodies, which appeared draped around the cell's nucleus, "chlamydozoa," derived from the Greek word for "cloak."1
One hundred years later, Chlamydia is the most common sexually transmitted bacterial disease in the world,2 with approximately 4 million new infections occurring in the United States annually.3 A recent study conducted by the Centers for Disease Control and Prevention reported that approximately 26 percent of adolescent females (age 14 to 19) in the United States have at least one of the four most common sexually transmitted diseases. This study, the first national investigation of the prevalence of the most common STDs among young women, reported a 4-percent rate of Chlamydia, placing it as the second most prevalent STD in this population.4 Perhaps unexpectedly, Chlamydial infections can manifest in the eye, in the forms of inclusion conjunctivitis and trachoma, although each involves a different subtype of the bacteria. Inclusion conjunctivitis typically occurs in developed countries; in contrast, trachoma is primarily restricted to impoverished regions. While involving different serotypes, both diseases are caused by the same species of Chlamydia, illustrating the indiscriminate impact of C. trachomatis.
Chlamydia the Bacteria
The bacteria family Chlamydiacea includes nine different species and can infect many other animals besides humans.5 C. trachomatis is responsible for the human forms of chlamydial ocular infections—inclusion conjunctivitis and trachoma. Testing of polyclonal and monoclonal antibodies with major outer-membrane protein has identified 19 different human serotypes and several variants of C. trachomatis.6 Serotypes D, Da, E, F, G, H, I, Ia, J, and K are associated with inclusion conjunctivitis (as well as genital infections), while serotypes A, B, Ba, and C are usually isolated from trachoma.7
Approximately 75 percent of C. trachomatis infections in women and half in men are asymptomatic,8 and when they do manifest, symptoms don't appear for several weeks.9 Chlamydia has been associated with bacterial vaginosis,10 and concurrent gonorrhea has been shown to reactivate latent chlamydial infection.11 Additionally, chlamydial ocular infections have also been associated with concomitant syphilis.12 Chlamydia can occur more often in patients with other STDs, but not to a statistically significant degree.13,14 Even so, engaging in sexual activity with casual or multiple partners and/or partners with urogenital complaints, as well as the presence of urethritis symptoms, are predictors of Chlamydia.15 Genital chlamydial disease often leads to urethritis, epididymitis and prostatis in men. In women, cervicitis and pelvic inflammatory disease can ensue, and if left untreated, the latter can cause complications such as ectopic pregnancy, tubal infertility and chronic pain.16
Inclusion Conjunctivitis
Here are the variants of inclusion conjunctivitis and ways to diagnose them:
• Adult inclusion conjunctivitis. The ocular manifestation of Chlamydia ostensibly stems from poor personal hygiene and the transmission of contaminated genital secretions to the eye, either via autoinoculation or from a sexual partner. Indeed, various diagnostic tests revealed 40 to 90 percent of an adult population with inclusion conjunctivitis to also have genital chlamydia.17 In a study of adult conjunctivitis, Chlamydia accounted for 19 percent of the investigated cases.18 Repeated ocular infection is rare, but can lead to corneal scarring.7
Signs and symptoms of adult inclusion conjunctivitis include mucopurulent discharge, lid swelling, irritation,12 foreign body sensation, redness, an enlarged preauricular lymph node and diffuse mixed papillary follicular conjunctivitis. Superficial vascularization, peripheral scarring, superficial punctate epithelial defects and peripheral subepithelial infiltrates can occur if the disease is left untreated. Adult inclusion conjunctivitis is usually self-limiting. If the infection doesn't resolve, the lack of antibiotic therapy can allow the disease to last for years, although it won't advance beyond peripheral corneal involvement.2
• Neonatal inclusion conjunctivitis. Chlamydia-infected mothers can pass the infection on to their newborns during parturition. Although prevalent two decades ago,19,20 the rate of chlamydial infections among infants born per vaginum appears to be decreasing, as more women are screened and treated.21 Neonatal inclusion conjunctivitis due to C. trachomatis presents within the first three weeks of life and is usually self-limiting.22 Mucous discharge, redness, lid swelling and diffuse papillary conjunctivitis are among the signs of chlamydial disease. Newborns don't present with follicles.2 Persistent or untreated disease will result in the occasional corneal micropannus and palpebral conjunctival scarring; the infection can spread to the nasopharynx and lower respiratory tract and lead to pneumonia if untreated.22
• Diagnosis and treatment of inclusion conjunctivitis. Chlamydial conjunctivitis is detectable by a cytological examination of stained smears from tissue. Trachoma and acute chlamydial conjunctivitis can be detected by Giemsa staining of conjunctival cells, which also reveals intracellular inclusion bodies. Unfortunately, this technique is an insensitive tool for adult chlamydial conjunctivitis detection, but it is very sensitive for infants.7 Immunofluorescent antibody staining of chlamydial antigen and nucleic acid amplification of conjunctival smears are newer and more accurate techniques.2,23,24 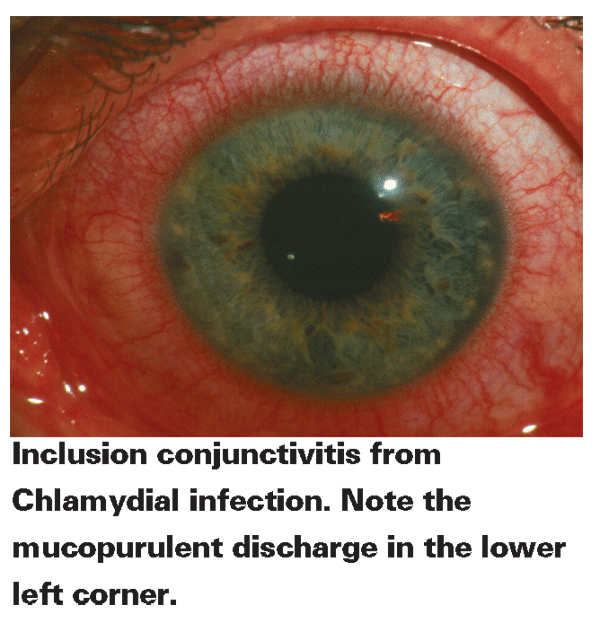
Recommended treatment for inclusion conjunctivitis includes both topical and systemic antibiotics. A two-to-three week regimen of erythromycin or tetracycline ointment with systemic tetracycline, doxycycline or erythromycin is the usual treatment for adult chlamydial conjunctivitis.12 A single oral dose of azithromycin has been shown to control ocular redness and mucous discharge.25 Furthermore, this dosing azithromycin is as effective as 10-day doxycycline therapy in the eradication of C. trachomatis.26 Infected mothers and their partners should take systemic tetracyclines, macrolides or azolides. Infants are usually treated with erythromycin ophthalmic ointment for one week and erythromycin or azithromycin elixir for two to three weeks.2
Trachoma
Repeated infection with gram negative C. trachomatis results in trachoma, a chronic, inflammatory, follicular form of keratoconjunctivitis. Approximately 84 million individuals throughout the world have active trachoma,27 and 8 million have become blind from it.28 In 2002, trachoma accounted for about 3.6 percent of worldwide blindness,29 and it's one of the leading causes of infectious blindness.2
The disease primarily occurs in underdeveloped nations that lack the medical services needed to combat repeated chlamydial infections. It's difficult to determine which personal habits or conditions increase the risk of trachoma, but some of the greatest household risks include the presence of several young children, insufficient access to water, poor sanitation and hygiene, and high fly densities.27 Chandler R. Dawson, MD, of the University of California, San Francisco, who has been involved in trachoma research for more than 40 years, also found crowding within a home increased the risk.30 Endemic trachoma has been correlated with seasonal epidemics of bacterial (Hemophilus, pneumococcus or Moraxella) conjunctivitis, which contribute to the severity of the ocular infection.31
As other severe ocular diseases such as severe papillary conjunctivitis can be misdiagnosed as trachoma, cytological assessments are imperative for an accurate diagnosis. Several weeks pass before the first clinical signs, as these result from inflammation. The median time course of disease in 0- to 4-year-olds is 13.2 weeks, and in patients older than 15 years of age, 1.7 weeks.9
The definitive sign of trachoma is follicles on the tarsal conjunctiva. Papillae form between the follicles, and in severe cases, the papillae join, making the conjunctiva appear thickened and velvety. Other signs include mucopurulent ocular discharge and scarred limbal follicles. Follicles at least 0.5 mm in size on the upper tarsal conjunctiva, and inflammatory thickening obscuring more than half of the normal deep tarsal vessels, are signs of active disease, with the latter representing severe disease. Recurring trachoma leads to the presentation of Arlt's line, easily visible white bands of scars, which are initially scarce but form a thick, basket-weave pattern with time. Significant scarring contracts the tarsal conjunctiva, leading the lid margin to roll towards the eye and subsequent trichiasis. The rubbing of the lashes on the cornea is painful, and if left untreated, rapid scarring and opacification ensues, resulting in blindness. Scarring, trichiasis and corneal opacity are the signs of cicatricial trachoma, the last stage.27
Treating Trachoma SAFE-ly
In July 1996, the World Health Organization first proposed the SAFE strategy—Surgery, Antibiotics, Facial cleanliness, and Environmental improvements—as a treatment for trachoma.27 Currently, the most effective therapy for trachoma is trichiasis surgery; although the procedure doesn't restore vision, it still must be performed soon after diagnosis to reduce discomfort.32 Surgery is the only valuable therapy for individuals suffering from trichiasis but, unfortunately, many of these patients don't undergo the procedure because of the high recurrence rate (8 to 18 percent after one or two years, 60 percent after three years), lack of knowledge, fear, perceived costs and transportation difficulties. Trichiasis surgery is also important for the elimination of trachoma, as there is a time lag between the active disease in children and trachomatous trichiasis in adults: Even if the entire world were rid of active trachoma today, surgery would still have to be performed for another 30 years to eradicate the disease.27
Antibiotics comprise another component of the SAFE strategy, and several studies reveal that oral azithromycin is the most effective antibiotic therapy.33-35 This allows a single dose or single daily dose regimen to be an effective dosing regimen for azithromycin, which is likely to improve patient compliance.36 For communities in which the prevalence of trachoma is greater than 10 percent in children aged 1 to 9 years old, the WHO recommends all members take oral azithromycin annually for at least three years. A single dose of azithromycin yields better compliance than topical tetracycline, which calls for a six-week dosing regimen.27 Also, the practice of supplementing topical treatment with systemic antibiotics in children with severe trachoma isn't only cost-effective, but also improves the disease.37 Azithromycin (ranging from one to six doses) was also shown to be as effective as oxytetracycline/ polyxin eye ointment (q.i.d. for five days every four weeks for a total of six treatment cycles) among children in rural Egypt.38 Furthermore, azithromycin also has a low potential for resistance in Chlamydia and other bacteria, and yields limited side effects, making it a preferable treatment option. The optimal dosing schedule of repeated mass treatment remains inconclusive, but it has been suggested that six monthly treatments are more effective than an annual retreatment.27
The third element of the SAFE strategy is facial hygiene. Children residing in the same household can transfer the disease to each other via contaminated washcloths.27 Furthermore, trachoma can be transmitted by flies.2 Lastly, environmental improvements such as provisions of water, latrines, refuse dumps, insecticide spray, relocation of animal pens, and health education comprise an important component of the SAFE strategy. One study, however, shows that environmental advances, when added to antibiotic therapy, did not diminish the number of trachoma cases.27
Although the rate of neonatal chlamydial conjunctivitis is low (eight out of 1,000 births)39 and C. trachomatis accounts for less than 25 percent of chronic conjunctivitis cases in the United States,18 trachoma afflicts 84 million individuals worldwide.27 Inclusion conjunctivitis and trachoma might be caused by different serotypes of C. trachomatis, but the presence of each disease in different areas throughout the globe demonstrates the bacteria's ability to indiscriminately transcend race, class and age. Given that Chlamydia impacts so much of the world's population in a variety of ways, clinicians and epidemiologists must collaborate on the development of optimal therapies for both ocular chlamydial diseases so we may see the eventual elimination of each type of infection.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Baguley S, Greenhouse P. Non-genital manifestations of Chlamydia trachomatis. Clin Med 2003;3:3:206-8.
2. Kalayoglu MV. Ocular chlamydial infections: Pathogenesis and emerging treatment strategies. Curr Drug Targets Infect Disord 2002;2:1:85-91.
3. Darville T. Chlamydia trachomatis infections in neonates and young children. Semin Pediatr Infect Dis 2005;16:4:235-44.
4. Center for Disease Control and Prevention. Nationally representative CDC study finds 1 in 4 teenage girls has a sexually transmitted disease. CDC press release. March 11, 2008. Available at http://www.cdc.gov/stdconference/2008/media/release-11march2008.pdf. Accessed March 17, 2008.
5. Bush RM, Everett KD. Molecular evolution of the Chlamydiaceae. Int J Syst Evol Microbiol 2001;51(Pt 1):203-20.
6. Ngandjio A, Clerc M, Fonkoua MC, et al. Restriction endonuclease patterns of the omp1 gene of reference Chlamydia trachomatis strains and characterization of isolates from Cameroonian students. J Med Microbiol 2004;53(Pt 1):47-50.
7. Thomas JM, Heggie AD, Lass JH. Chlamydial Disease. In: Albert DM, Jakobiec FA, eds. Principles and Practice of Ophthalmology.
8. Shah AP, Smolensky MH, Burau KD, et al. Recent change in the annual pattern of sexually transmitted diseases in the
9. Lansingh VC, Carter MJ. Trachoma surveys 2000-2005: Results, recent advances in methodology, and factors affecting the determination of prevalence. Surv Ophthalmol 2007;52:5:535-46.
10. Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis 2007;196:11:1692-7.
11. Batteiger BE, Fraiz J, Newhall WJ, et al. Association of recurrent chlamydial infection with gonorrhea. J Infect Dis 1989;159:4:661-9.
12. Avery RK, Sullivan Baker A. Chlamydial Disease. In: Albert DM, Jakobiec FA, eds. Principles and Practice of Ophthalmology.
13. Bagshaw SN, Edwards D. Risk factors for Chlamydia trachomatis infection of the cervix: A prospective study of 2000 patients at a family planning clinic. N Z Med J 1987;100:827:401-3.
14. McCormack WM, Rosner B,
15. Joffe GP, Foxman B, Schmidt AJ, et al. Multiple partners and partner choice as risk factors for sexually transmitted disease among female college students. Sex Transm Dis 1992;19:5:272-8.
16. Navarro C, Jolly A, Nair R, Chen Y. Risk factors for genital chlamydial infection. Can J Infect Dis 2002;13:3:195-207.
17. Stenberg K, Mardh PA. Genital infection with Chlamydia trachomatis in patients with chlamydial conjunctivitis. Sex Transm Dis 1991;18:1:1-4.
18. Rapoza PA, Quinn TC, Terry AC, et al. A systematic approach to the diagnosis and treatment of chronic conjunctivitis. Am J Ophthalmol 1990;109:2:138-42.
19. Schachter J, Grossman M, Holt J, et al. Prospective study of chlamydial infection in neonates. Lancet 1979;2:8139:377-80.
20. Schachter J, Grossman M, Sweet RL, et al. Prospective study of perinatal transmission of Chlamydia trachomatis. Jama 1986;255:24:3374-7.
21. Hammerschlag MR, Gelling M, Roblin PM, et al. Treatment of neonatal chlamydial conjunctivitis with azithromycin. Pediatr Infect Dis J 1998;17:11:1049-50.
22. Thomas J, Heggie A, Lass J. Chlamydial Disease. In: Albert DM, Jakobiec F, eds. Principles and Practice of Ophthalmology.
23. Lin J, Li Y, Zhang J, et al. Rapid diagnosis of chlamydial conjunctivitis in laboratory. Yan Ke Xue Bao 1999;15:3:191-4.
24. Malathi J, Madhavan HN, Therese KL, Joseph PR. A hospital based study on the prevalence of conjunctivitis due to Chlamydia trachomatis. Indian J Med Res 2003;117:71-5.
25. Salopek-Rabatic J. Chlamydial conjunctivitis in contact lens wearers: Successful treatment with single dose azithromycin. Clao J 2001;27:4:209-11.
26. Katusic D, Petricek I, Mandic Z, et al. Azithromycin vs. doxycycline in the treatment of inclusion conjunctivitis. Am J Ophthalmol 2003;135:4:447-51.
27. Wright HR, Turner A, Taylor HR. Trachoma and poverty: Unnecessary blindness further disadvantages the poorest people in the poorest countries. Clin Exp Optom 2007;90:6:422-8.
28. Atik B, Thanh TT, Luong VQ, et al. Impact of annual targeted treatment on infectious trachoma and susceptibility to reinfection. Jama 2006;296:12:1488-97.
29. Resnikoff S, Pascolini D, Etya"ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004;82:11:844-51.
30. Dawson CR, Daghfous T, Messadi M, et al. Severe endemic trachoma in
31. Dawson CR, Schachter J. Editorial: Trachoma—antibiotics or vaccine? Invest Ophthalmol 1974;13:2:85-6.
32. Bowman RJ. Trichiasis surgery. Community Eye Health 1999;12:32:53-4.
33. Bowman RJ, Sillah A, Van Dehn C, et al. Operational comparison of single-dose azithromycin and topical tetracycline for trachoma. Invest Ophthalmol Vis Sci 2000;41:13:4074-9.
34. Fraser-Hurt N, Bailey RL, Cousens S, et al. Efficacy of oral azithromycin versus topical tetracycline in mass treatment of endemic trachoma. Bull World Health Organ 2001;79:7:632-40.
35. Schachter J,
36. Peters DH, Friedel HA, McTavish D. Azithromycin. A review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs 1992;44:5:750-99.
37. Dawson CR, Schachter J. Strategies for treatment and control of blinding trachoma: Cost-effectiveness of topical or systemic antibiotics. Rev Infect Dis 1985;7:6:768-73.
38.
39. Yip TP, Chan WH, Yip KT, et al. Incidence of neonatal chlamydial conjunctivitis and its association with nasopharyngeal colonisation in a



