|
Ivan J. Suñer, MD |
As age-related macular degeneration primarily affects the macula, it has a severe impact on many of the basic activities of daily living such as driving, recognizing faces and reading. Therefore, it robs affected individuals of their independence in their retirement years. Fifteen million people in the United States alone are affected by AMD, and current estimates project this figure to increase by 50 percent by the year 2020. Approximately 1.75 million (10 percent) Americans have the advanced or late forms of the disease, exudative/wet AMD and geographic atrophy.1
Epidemiology of Smoking and AMD
AMD is a multifactorial disease with associated age, environmental, systemic and genetic factors. Age is the major risk factor in AMD. The prevalence of all forms of AMD increases significantly with age. It affects approximately 17 percent of all individuals between the ages of 55 and 64, and the prevalence rises to approximately 37 percent in those 75 or older.2 The more advanced, disabling forms (exudative/wet and geographic atrophy) affect 1 percent of Caucasian patients in their 50s, rising to more than 15 percent of those in their 80s.1
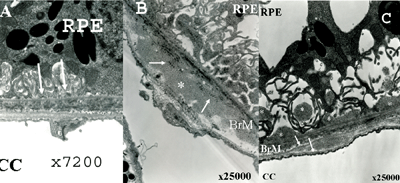 |
| Figure 1. Representative transmission electron micrographs (TEM) of outer retina and choroid in 16-month-old female mice demonstrating dry AMD findings in mice exposed to cigarette smoke or hydroquinone, a major component of cigarette tar. Panel A. TEM of mouse fed high fat diet without exposure to cigarette smoke or hydroquinone; specimen shows mild nodular sub-RPE deposits (white arrows); Bruch's membrane and choriocapillaris are normal thickness. Panel B. TEM of mouse fed high-fat diet and exposed to cigarette smoke; there are sub-RPE deposits (white arrows) and thickening of Bruch's membrane with accumulation of a homogenous material (white asterisk). Panel C. TEM of mouse fed high-fat diet plus hydroquinone; there are thick sub-RPE deposits (white asterisks) and marked thickening of Bruch's membrane (white arrows). |
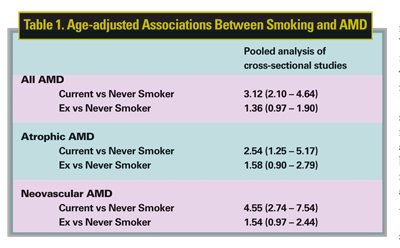
Cigarette smoking is the single most important modifiable environmental risk factor for development of all forms of AMD. Multiple large, well-controlled, cross-sectional3-6 and prospective7,8 studies in the United States, Australia, France and the Netherlands have demonstrated a 2.5- to threefold increase in the risk of all forms of AMD in smokers. Of particular interest is an analysis of pooled data from cross-sectional studies carried out in North America, Europe and Australia. This data shows an especially large disparity between current and never smokers with a threefold increase in all forms of AMD, 2.5-fold increase in atrophic AMD, and 4.5-fold increase in neovascular AMD9 (See Table 1).
 |
| Figure 2. Representative flatmount preparation (propidium iodide stain) of the posterior pole of 11-month-old mouse eyes 4 weeks after laser treatment. Four laser spots centered on the optic nerve (D) were performed. Panel A. Control group. CNV lesions were small and circular with discrete borders (dotted lines). Panel B. Oral nicotine group. In this example there is coalescence of three laser injuries gives rise to a large CNV complex (dotted lines). Panel C. Oral nicotine and subconjunctival hexamethonium group. A reduction of the severity of CNV lesions can be clearly appreciated with concurrent administration of a nicotine antagonist, hexamethonium. CNV lesions (dotted line) were similar in size to the control. |
An important factor, yet difficult to tease out of this association, is the influence of pack years, current smoking status, ex-smoker and passive smoker. A recent study looked at these issues specifically.10 It demonstrated a strong association between AMD and number of pack years smoked. The odds ratio in patients smoking greater than 40 pack years was 2.75 as compared to nonsmokers; the odds ratio was 3.43 for geographic atrophy and 2.49 for choroidal neovascularization. Smoke cessation was associated with decreasing risk, reaching a similar risk to nonsmokers at 20 years of not smoking. Passive smoking was associated with an odds ratio of 1.87.
An interesting observation is the trend towards earlier onset of AMD, including advanced forms, in Asian countries. This has been attributed in large part to the increased rates of smoking and environmental pollutants.11
Why Study Smoking and AMD?
Despite the wealth of robust epidemiologic evidence associating smoking with AMD, there is a relative dearth of science to support it. Only now are we beginning to study and elucidate pathobiologic mechanisms of this association. This may be partly due to the concept that cigarette smoking is considered purely a modifiable risk factor. In other words, patients with AMD should quit smoking, and, therefore, there is not more to be gained by further pursuing this association on a biologic level. However, studying the effects of smoking on AMD may provide us with insights into the pathobiology of this complex disease process. Furthermore, some of the lessons we learn may translate into therapies for smokers as well as for nonsmokers.
Composition of Cigarette Smoke
Cigarette smoke is a complex mixture of more than 4,000 chemical substances. Selecting which molecules to study within the daunting number of potential candidates is a difficult issue. The agents present in cigarette smoke are generally subdivided into particulate and gaseous phases.12 The major components of the particulate phase are tar and nicotine, whereas the gaseous phase is composed primarily of carbon monoxide, carbon dioxide and nitric oxide.
Various proposed mechanisms by which cigarette smoke may cause end-organ damage include direct effects from chemicals in the smoke, immune activation, secondary hypoxia from pulmonary damage, and secondary sequelae from smoking-induced vascular disease.
Dry AMD and Smoking
The exact pathogenesis of drusen remains an unresolved question. One paradigm, "the response to injury" hypothesis, proposes that the RPE cell is the target for specific injury stimuli, resulting in deposit accumulation.13
Cigarette smoke tar contains numerous pro-oxidant compounds within the quinone family.14 Within these, hydroquinone, a benzene derivative, is the most abundant quinone in cigarette tar. High levels are detected in the plasma and urine of smokers.14
Our group tested this hypothesis in cultured human RPE cells. Incubation of RPE cells with hydroquinone induced a specific injury response called nonlethal blebbing, a process that is proposed to be related to sub-RPE deposit formation.15 Furthermore, exposure to hydroquinone also resulted in decreased levels of matrix metalloproteinase-2 and increased levels of collagen IV as measured by zymography (Suñer I, et al. Invest Ophthalmol Vis Sci. 2004;45: ARVO E-Abstract 1810). This leads to a net decrease in extracellular matrix turnover, which would result in thickening of Bruch's membrane or formation of sub-RPE deposits.
This relationship was further studied in an animal model by exposing mice to whole cigarette smoke or oral hydroquinone. In mice fed a high-fat diet and exposed to either cigarette smoke in a smoking chamber or dietary hydroquinone, Bruch's membrane was thickened and sub-RPE deposits developed in contrast to controls only receiving a high-fat diet (See Figure 1).16 Therefore, smoke-related oxidants, specifically tar, appear to be a significant oxidative injury stimulus that leads to dry AMD in the context of other oxidative sources such as high-fat diet or blue light. It is also likely that while tar is a potent oxidant within cigarette smoke, it is not the only oxidant molecule in this pathway.
Wet AMD and Smoking
Nicotine is an attractive candidate molecule to explain an association of smoking with wet AMD. It has been shown to be mitogenic for vascular endothelial cells and smooth muscle pericytes, to reduce apoptosis of vascular endothelial cells, and to induce the formation of capillary tubes.17,18
We explored the effects of nicotine in a laser model for CNV in mice. We compared CNV size in those receiving nicotine in the drinking water or cigarette smoke in a smoking chamber with control animals. Mice receiving nicotine or cigarette smoke had a statistically significant increase in CNV size (twice the diameter in aged mice). This effect was blocked by subconjunctival coadministration of a nonspecific nicotinic antagonist, hexamethonium (See Figure 2).17
The experiment was carried one step further, and bone-marrow transplantation was performed within the CNV mouse model. Mice that were exposed to cigarette smoke in a smoking chamber and subsequently had bone marrow ablation followed by bone marrow reconstitution from a control mouse had regression of CNV to control levels. Conversely, a control mouse receiving bone marrow from a cigarette smoke-exposed mouse demonstrated increased CNV size comparable to cigarette smoke-exposed mice (Cousins S, et al. Invest Ophthalmol Vis Sci. 2006;47:ARVO E-Abstract 4172). This suggests that bone marrow-derived cells, either endothelial or inflammatory precursor cells, may play a role in establishing or modulating choroidal neovascularization.
Immunohistochemical analysis of CNV in cigarette smoke-exposed or nicotine-exposed mice reveals an increased number of macrophages in these lesions as compared to controls. Furthermore, it demonstrated higher levels of macrophages expressing TNF-a and COX-2 (Suñer I, et al. Invest Ophthalmol Vis Sci. 2006;47: ARVO E-Abstract 1531). These are markers of activated macrophages, which supports current theories of inflammatory contributions to the pathogenesis of AMD.
Clinical Implications
The immediate clinical implications of these findings are that we must continue to impress upon our patients that smoking is not only a significant risk factor for cancer, heart disease and pulmonary disease, but that it is the leading modifiable risk factor for the leading cause of blindness in patients older than 50 years of age. It appears that risks correlate with total number of pack years, and that smoke cessation may result in risk reduction to that of never smokers at 20 years. Furthermore, second hand smoke also confers risk for AMD.
Dry AMD patients should be encouraged to quit smoking. Those with higher-risk lesions per the Age-Related Eye Disease Study should take the recommended vitamin supplementation with the exception of ß-carotene, which has been demonstrated to increase rates of lung cancer.20
Patients with active choroidal neovascularization should be especially encouraged to quit smoking. Furthermore, they should abstain from nicotine replacement therapies as nicotine may promote growth of the lesion.
Future Directions
We are entering an era of molecular therapies for retinal diseases. As we learn more about the pathobiologic mechanisms by which smoking impacts AMD, we may discover specific pathways of oxidation or immunomodulation in dry AMD and of vascular endothelial cell and smooth-muscle pericyte proliferation, matrix turnover or immunomodulation that are relevant to wet AMD.
It is also likely that these pathways will be relevant to the biology of AMD not only in smokers, but also nonsmokers. Taking the example of nicotine in wet AMD one step further, it may be that nicotinic receptors (which are also present in nonsmokers) may be a viable target for therapy.
Dr. Suñer is an associate professor of ophthalmology on the Retina Service at Duke University Eye Center and chief of ophthalmology, Durham Veterans Affairs Medical Center, Durham, N.C. Contact him at Duke University Eye Center, DUMC 3802 Erwin Road, Durham, N.C. 27710, 919-6868-1876 (office), 919-681-6474 (fax), or ivan.suner@duke.edu.
Dr. Cousins is a professor of ophthalmology and director of the Duke Center for Macular Diseases, Duke University Eye Center, Durham, NC. Contact him at Duke University Eye Center, DUMC 3802 Erwin Road, Durham, NC 27710, 919-684-3090 (office), 919-681-6474 (fax), or scott.cousins@duke.edu.
1. The Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564-572.
2. Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99:933-943.
3. Klein R, Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: The relation of age-related maculopathy to smoking. Am J Epidemiol 1993;137:190-200.
4. Vingerling JR, Hofman A, Grobbee DE, de Jong PT. Age-related macular degeneration and smoking. The Rotterdam Study. Arch Ophthalmol 1996;114:1193-1196.
5. Delcourt C, Diaz JL, Ponton-Sanchez A, Papoz L. Smoking and age-related macular degeneration. The POLA Study. Pathologies Oculaires Liees a l"Age. Arch Ophthalmol 1998;116:1031-1035.
6. Smith W, Mitchell P, Leeder SR. Smoking and age-related maculopathy. The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:1518-1523.
7. Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996;276:1141-1146.
8. Christen WG, Glynn RJ, Manson JE, et al. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 1996;276:1147-1151.
9. Tomany SC, Wang JJ, van Leeuwen R, Klein R, Mitchell P, et al. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology 2004;111:1280-1287.
10. Khan JC, Thurlby DA, Shahid H, et al. Smoking and age-related macular degeneration: The number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularization. Br J Ophthalmol 2006;90:75-80.
11. Tamakoshi A, Yuzawa M, Matsui M, et al. Smoking and neovascular form of age related macular degeneration in late middle-aged males: findings from a case-control study in Japan. Research Committee on Chorioretinal Degenerations. Br J Ophthalmol. 1997;81:901-904
12. Smith CJ, Hansch C. The relative toxicity of compounds in main¬stream cigarette smoke condensate. Food Chem Toxicol 2000;38:637–646.
13. Cousins SW, Espinosa DG, Alexandriou A, et al. The role of aging, high fat diet, and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res 2002;75:543-553.
14. Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 1997;105:875-882.
15. Strunnikova N, Zhang C, Teichberg D, Cousins SW, Baffi J, Becker KG, Csaky KG. Survival of retinal pigment epithelium after exposure to prolonged oxidative injury: a detailed gene expression and cellular analysis. Invest Ophthalmol Vis Sci 2004;10:3767-77.
16. Espinosa-Heidmann DG, Suñer IJ, Catanuto P, et al. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of Dry AMD. Invest Ophthalmol Vis Sci 2006;47:729-37.
17. Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84:2089-2098.
18. Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med 2001;7:833-839.
19. Suñer IJ, Espinosa-Heidmann DG, Marin-Castaño ME, Hernandez EP, Pereira-Simon S, Cousins SW. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2004;45:311-317.
20. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol 2001;119:1417-1436.



