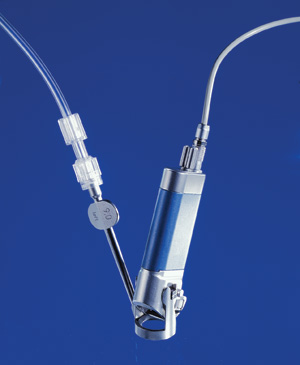
Schwind says its microkeratome offers quality and safety with an extremely low standard deviation of almost 12 µm, smooth surface cut quality, 360° free choice of hinge position and ergonomic design.
The microkeratome was developed in collaboration with the Columbian ophthalmic surgeon Dr. Cesar Carriazo and received its Food and Drug Administration approval in 2004. For more information, visit eye-tech-solutions.com.
 |
FDA Extended Wear Indication for Oasys
Vistakon reports that it received an additional indication for its Acuvue Oasys contact lenses with Hydraclear Plus. The approval is for up to six consecutive nights and seven days of extended wear.
The company says its senofilcon A silicone hydrogel material is 50-percent smoother than currently available silicone hydrogel lenses. These contact lenses also block greater than 96 percent of UVA rays and 99 percent of UVB rays, meeting the highest UV-blocking standards for contact lenses, according to the company.
For more information, call 1 (800) 843-2020 or visit ecp.acuvue.com.
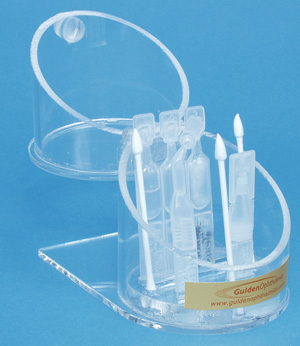
Time Dropper
Gulden Ophthalmics says its new Dropperette Caddie stores both opened and unopened dropperette containers, as well as surgical spears, in a sanitary organizer. The clear acrylic caddie can be opened with one hand, allowing doctors to hold the patient's eye open with the other.
Gulden Ophthalmics says its new item will be handy for doctors during postoperative care, such as instilling unpreserved NSAID or teardrops after LASIK, adjusting LASIK flaps with spears at the slit lamp, or instilling pressure-lowering drops after YAG laser.
For more information, visit guldenindustries.com.
 |
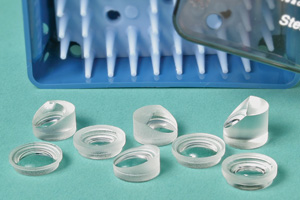
New Surgical Lenses from Volk
Volk Optical's ACS Direct Image lines of vitrectomy lenses include eight new lenses that provide clear direct views and can be safely steam-sterilized.
The company says the lenses eliminate the need for the use of a reinverter, and the low profile designs deliver high-resolution optics for clear views during surgery. The lenses fit in a standard suture ring, working seamlessly with current surgical methods. As part of Volk's AutoClaveSterilizable (ACS) collection, the lenses can endure repeated steam-sterilization.
The lenses are available in eight different styles: the Flat, HighMag 1.5x and Midfield for fundus viewing; the AFX AirFluid eXchange for visualization of the retina in an air-filled eye; the 15°, 30º and 45º Prisms for off-axis fundus viewing and views of the peripheral retina; and the 30º Midfield Prism for midperipheral pathology and laser applications.
A free 30-day trial is available from Volk. For information, call 1 (800) 345-8655 or visit volk.com.
 |
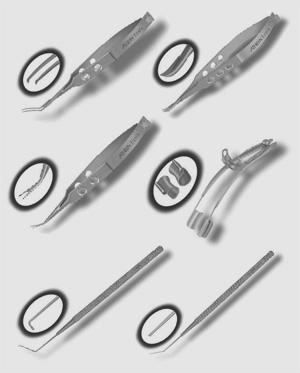
Rhein's New Single-Use Supplies
Rhein Medical has introduced the Rhein Eco's, stainless steel ophthalmic instruments that come packaged sterile, six per box, in double Tyvek pouches, and are ready for immediate use. The offering includes: capsulorhexis, corneal, and tying forceps; needle holders; scissors; manipulators; choppers; dilators and rotators; and speculums. Rhein says the instruments are rugged and ready for all types of anterior segment surgery.
Rhein has also published its new Ophthalmic Instrument Catalog, and it is available in print or CD form. The collection includes thousands of the most innovative and contemporary instrument designs in the world.
For a free copy of the catalogue or information on Rhein Eco's, contact Rhein Medical at 813-885-5050.
 |
Biofinity Cleared for U.S. Market
CooperVision has received FDA 510(k) clearance to market its Biofinity silicone hydrogel contact lenses for daily wear in the United States. The company is currently pursuing FDA clearance for overnight wear. U.S. availability is planned for July 2006.
CooperVision says the Biofinity lenses offer exceptional comfort due to the combination of high oxygen transmission, low modulus and higher water content. CooperVision says the material, comfilcon A, makes the lens inherently wettable, requiring no surface treatment or wetting agents, and provides a high level of oxygen transmissibility.
The Biofinity lens's patented, molded, rounded edge means less conjunctival interaction, which contributes to wearing comfort and enhanced ocular health, the company says. The aspheric front surface design reduces spherical aberration, thereby improving visual acuity, and the back surface design distributes pressure forces across the surface for additional wearing comfort. For more information, visit coopervision.com.



