Tumors of the ocular surface and the anterior segment of the eye may result from a wide range of conditions. Benign or malignant lesions may originate from several cell types, forming epithelial, melanocytic, lymphoid, fibrous and many other lesions.1 It’s important to determine the etiology and malignant potential of a lesion for appropriate diagnosis and treatment guidance. Tissue biopsy is the gold standard for establishing a diagnosis, but imaging-based modalities have been developed that can aid the clinician in assessing these tumors prior to histopathological confirmation.
The four most commonly used modalities are high-resolution anterior segment optical coherence tomography (HR-OCT), OCT angiography, ultrasound biomicroscopy and in-vivo confocal microscopy (IVCM).1-3 While useful, each technology has strengths and weaknesses that must be considered when examining ocular surface lesions. Here, we’ll highlight the pros and cons and discuss how these technologies can be incorporated in the evaluation of a patient with an ocular surface tumor.
High-resolution AS-OCT
Anterior segment optical coherence tomography provides high-resolution cross-sectional images of the anterior segment tissue through optical scattering created by low-coherence interferometry.4 Since its first iteration by David Huang, MD, PhD, and colleagues, HR-OCT technology has progressed to resolutions of 5 to 10 µm and scan depths up to 2 to 7 mm. Ultrahigh-resolution anterior segment OCT provides even higher resolution of 2 to 5 µm.5-7
 |
|
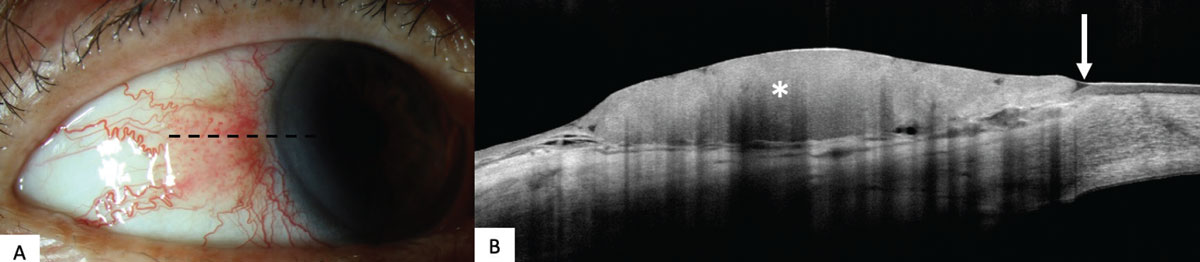
Figure 1. (A) Clinical slit lamp photo of ocular surface squamous neoplasia on the temporal conjunctiva of the right eye with papillomatous, gelatinous and opalescent features. (B) High-resolution anterior segment optical coherence tomography image of ocular surface squamous neoplasia demonstrating classic features of a hyperreflective and thickened epithelium (asterisk) and an abrupt transition between abnormal and normal epithelium (arrow). |
• Advantages. In today’s ophthalmic practice, HR-OCT provides an in-office, non-invasive diagnostic modality with readily available results, allowing for an “optical biopsy” that often mirrors the pathologic changes seen on histopathology.5,7,8 This is useful in clinical decision making. For example, in a lesion with classic clinical and OCT features of ocular surface squamous neoplasia (OSSN), treatment with topical chemotherapy may be initiated. In a lesion with features of melanoma, the surgeon should defer an incisional biopsy and instead plan a treatment with an excision biopsy with wide margins and adjuvant cryotherapy.
Anterior OCT is most useful for differentiating epithelial lesions from subepithelial ones. For example, when evaluating a pigmented conjunctival lesion, the detection of thickened hyperreflective epithelium, with an abrupt transition from normal to abnormal epithelium, points towards a diagnosis of pigmented OSSN as opposed to a conjunctival melanoma (CM).8 Conversely, the clinician can identify that a non-pigmented conjunctival lesion with a thin epithelium and a lesion noted under the epithelium is most likely not an OSSN and, if there’s a hyperreflective subepithelial mass, that it may be an amelanotic melanoma.
Furthermore, HR-OCT images can detect or rule out the presence of tumors, particularly OSSN, in eyes with co-morbidities, such as limbal stem cell deficiency or a history of herpetic keratitis.9 HR-OCT can also help the clinician monitor treatment response, since it can detect subclinical disease. This helps avoid early termination of topical chemotherapy.2 HR-OCT has also been used preoperatively to improve tumor visualization and provide a blueprint prior to surgical excision and direct biopsy localization.10 Fortunately, there’s a short learning curve for interpreting images. Even novice users can correctly identify a variety of ocular surface lesions with moderate sensitivity and high specificity.1,11
• Disadvantages. HR-OCT can be limited by shadowing in thick, keratinized or pigmented lesions. As such, the features and depth of these lesions are difficult to assess.12 Furthermore, HR-OCT isn’t able to examine tissue on a cellular level, and thus it can’t identify cellular atypia or invasion into deeper structures. The former limits its ability to distinguish squamous metaplasia from OSSN, complexion-associated melanosis (CAM) from primary acquired melanosis (PAM) and conjunctival lymphoma from benign reactive lymphoid hyperplasia (BRLH). While sometimes in squamous cell carcinoma a connection to the underlying subepithelial mass is visualized, the ability to evaluate invasion isn’t reliable.12-14 Ultimately, tissue is needed to examine structures on a cellular level and distinguish between these entities.2,15
 |
|
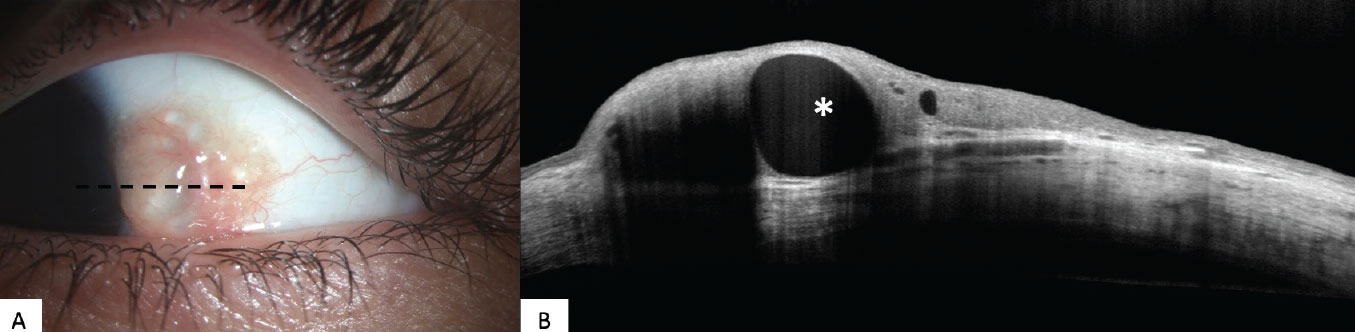
Figure 2. (A) Clinical slit lamp photo of a longstanding non-pigmented conjunctival nevus on the temporal aspect of the left eye. (B) High-resolution OCT image of a conjunctival nevus with characteristic features of an intralesional cyst (asterisk). |
• Imaging findings. Numerous tumors that arise on the ocular surface have distinguishing features on HR-OCT. OSSN is characterized by a thickened and hyperreflective epithelium with an abrupt transition between normal and abnormal epithelium (Figure 1).16 Using a custom-built, ultra-high-resolution OCT machine, with 2-3 µm of resolution, we found that an epithelial thickness of greater than 142 µm distinguished OSSN from pterygia with 94-percent sensitivity and 100-percent specificity.17,18 Further studies by our group with a commercially available HR-OCT (RTVue, Optovue, Fremont, California) showed a sensitivity and specificity of 100 percent with an epithelial thickness cut-off of 120 µm for differentiating OSSN and pterygia.14 More recently, it was shown that another commercially available device (Spectralis SD-OCT, Heidelberg Engineering, Heidelberg, Germany) can demonstrate a sensitivity and specificity of 100 percent for distinguishing OSSN from pterygia, with a cut-off of 141 µm.18
Melanocytic lesions also have several distinguishing features on HR-OCT. Intralesional cysts are a helpful feature that point towards a conjunctival nevus (Figure 2). In addition, basal epithelium hyperreflectivity is a feature shared by conjunctival CAM and PAM.14,19 A differentiating feature of nevi is the lack of cysts seen in CAM and PAM.12-14 CM on HR-OCT generally has a thin, uniform hyperreflective epithelium and, similar to PAM, lacks cysts. Unlike PAM, CM has a subepithelial hyperreflective mass and greater intensity of posterior shadowing (Figure 2C).14,20 Conjunctival lymphoma appears as a homologous, hyporeflective, subepithelial lesion surrounded by a hyperreflective band of tissue superior to the mass. BRLH appears similar to conjunctival lymphoma but has more of a granular appearance, with small, hyperreflective stippled dots within the mass.14,21
OCT Angiography
OCTA non-invasively collects numerous scans at a wide range of angles to create a three-dimensional image of the anatomy, vasculature and blood flow of the anterior segment.2,22-26 OCTA was originally designed to evaluate retinal pathology; it requires an adapter lens to image the anterior segment and the tumors that may arise in the iris, cornea, conjunctiva and sclera.26,27
• Advantages. OCTA is a relatively recent technology with the first report of OCTA used in the anterior segment in 2015.28 As a non-invasive tool for imaging vascular tissue, it has the benefit of no side effects or reactions secondary to injected dyes used in traditional angiography.29,30 OCTA is also able to detect certain features of OSSN that can’t be detected clinically or with HR-OCT—most notably vascularization patterns within, adjacent to and under the tumor.31
• Disadvantages. A limitation of OCTA is that it produces images with artifacts, which can limit interpretation of the image. The most prevalent types of artifacts are associated with eye motion, projections and low OCT signal.22-24 The frequency and severity of artifacts, such as segmentation artifacts, may depend on the OCTA device used, while others such as eye motion may depend on patient factors (e.g., excessive eye movements in a patient with Parkinsonism).24 Awareness of potential artifacts is key to interpreting OCTA images. Hopefully, future software and faster scanning will ameliorate some of these issues.
 |
|
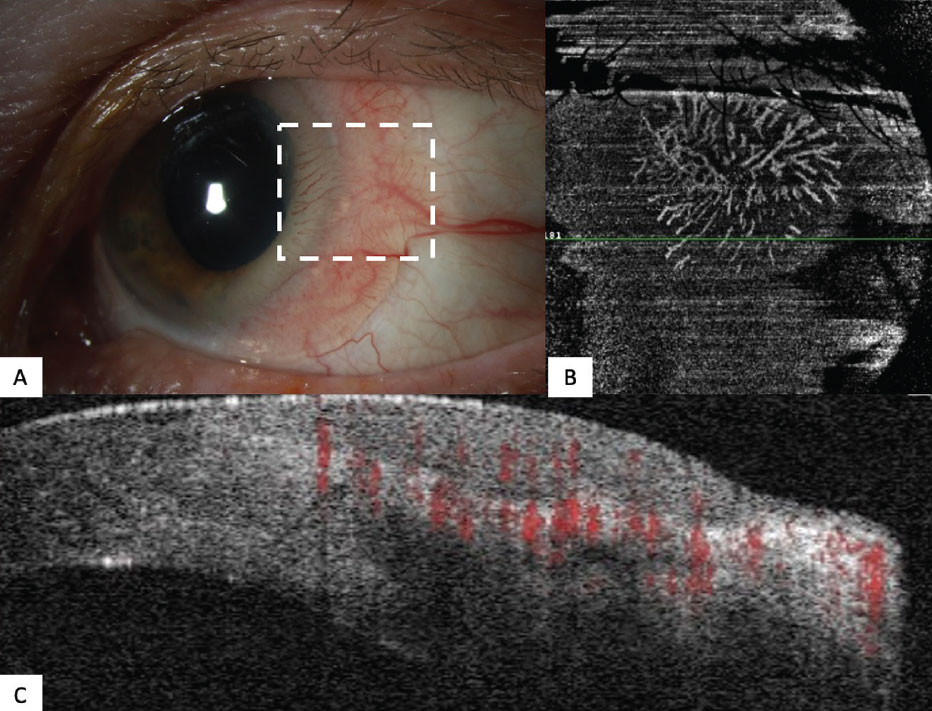
Figure 3. (A) Clinical slit lamp photo of an ocular surface squamous neoplasia on the temporal conjunctiva of the left eye with opalescent and gelatinous features. (B) OCTA image (dashed box) displaying a superficial segment of the OSSN demonstrating the vascular “sea fan” architecture of the tumor. (C) B-scan of the OSSN with zones highlighted in red demonstrating the vascular flow characteristics of the lesion. Note the thickened hyperreflective epithelium. |
• Imaging findings. Compared to HR-OCT, the literature on ocular surface tumor findings with OCTA is limited. However, one case series comparing OCTA findings in two OSSN lesions vs. one pterygium lesion described a highly “zigzag vessel pattern” in OSSN, compared to a “straight vessel pattern” in the pterygium.31 One study used OCTA to determine tumor total density (percent of blood vessels within the entire tumor) and vessel area density (VAD, percent of blood vessels within 2.14 mm2) in the tumor, the surrounding tissue and the contralateral eye.32 It found that VAD was highest within the conjunctival tumors, followed by the subepithelial tissue adjacent to tumors and then by tissue 200 µm below the tumor. Another group examined features of melanocytic lesions on OCTA and reported more vessel tortuosity in CM compared to PAM and conjunctival nevi.33 While these features haven’t been used to diagnose a lesion, they do provide adjuvant findings that aren’t always apparent clinically.
OCTA’s use on ocular surface tumors is still in its infancy, and it’s not yet clear how the detection of the vessels will help with diagnosis and monitoring.8,21,24,26 Nevertheless, it provides highly detailed images that aren’t clinically apparent, and it’s currently helping to elucidate the pathophysiology of tumorigenesis (Figure 3).
Ultrasound Biomicroscopy
UBM applies high-frequency ultrasound waves to biological tissue such as the anterior segment to obtain a cross-sectional image.1-3,34 Quantitative A-scan (lesions >2 mm) and standardized B-scan measurements related to anatomy and pathology of the conjunctiva, cornea, iridocorneal angle, iris, zonules, ciliary body and lens can be obtained using UBM.34 Both 25 MHz and 50 MHz ultrasound waves can be used to examine the ocular surface. Higher frequencies (50 MHz) provide better resolution of the anatomy from the anterior chamber to the capsular area, and lower frequencies (25 MHz) provide lower resolution but wider and deeper fields of view of structures spanning the cornea to the retrocapsular area.1-3,34
• Advantages. The primary benefit of UBM is its ability to penetrate opaque tumors and partially overcome posterior shadowing, which commonly affects HR-OCT. It also provides high-resolution imaging of dense and thick lesions.1-3 UBM has been shown to be superior to HR-OCT, in terms of visualizing tumor margins. In a case series of 200 anterior segment tumors, UBM demonstrated mild superiority in identifying the anterior margin (90 vs. 82 percent) and significant superiority in identifying the posterior margin (90 vs. 29 percent) of the lesion.35 Due to its ability to effectively probe large lesions, UBM is an excellent tool to assess for occult intraocular invasion and metastasis, which can present as blunting of the anterior chamber angle and uveal thickening.2,3 With respect to melanocytic tumors that are often thick and pigmented, UBM can often image the deep margin.1,2 In this regard, UBM has potential as a non-invasive tool for estimating tumor thickness prior to surgical intervention. A small case series demonstrated the relative agreement (difference of 0- to 0.5-mm thickness) compared to Breslow thickness.36
 |
|
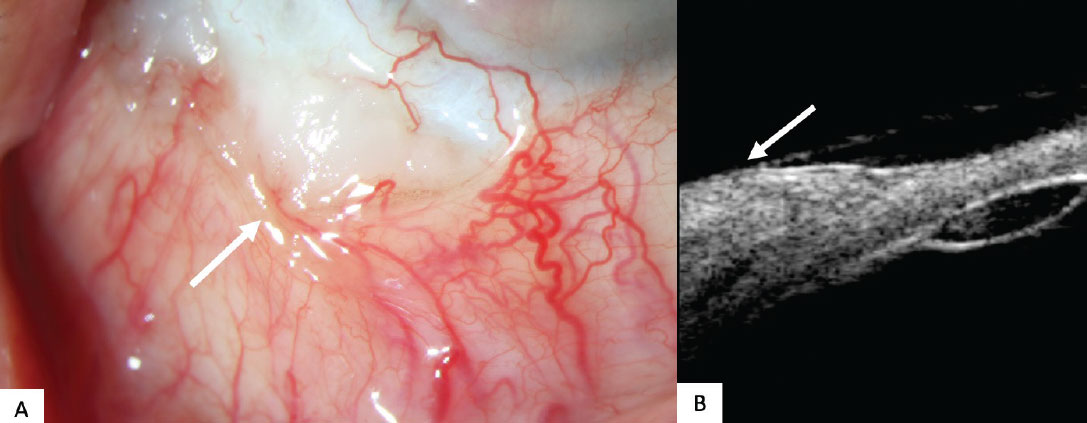
Figure 4. (A) Clinical slit lamp photo of a squamous cell carcinoma on the inferotemporal conjunctiva of the right eye (arrow). Patient is post prior surgical resection. (B) Ultrasound biomicroscopy 50 MHz B-scans demonstrating a dome-shaped, well-outlined lesion with an irregular internal appearance and mildly echoic features over the temporal sclera (arrow). |
• Disadvantages. While UBM can often delineate the margins and extent of large tumors, it doesn’t yield high-resolution views of the interior aspects of the lesion and often can’t visualize thin tumors well.1 It’s also unable to differentiate among different tumors, as HR-OCT can (Figure 4). Furthermore, UBM, unlike HR-OCT and OCTA, requires an eyebath in a reclined position and tissue contact, and the machine requires more technical familiarity.1-3 Access to UBM is generally limited to large tertiary centers.
• Imaging findings. On UBM, the stromal component and the intraocular extension of the tumors (if present) have variable echogenicity.2,37 This is true for a variety of tumors and, as such, no specific, unique intralesional findings have been described that distinguish OSSN from CM.35 In addition, there’s a lack of literature describing the characteristics of conjunctival lymphoma on UBM. However, intraocular lymphomas have been described as hypoechoic in the anterior and posterior chambers of the eye.38 Future studies are needed to best delineate the internal echoic features of conjunctival tumors on UBM.
In-Vivo Confocal Microscopy
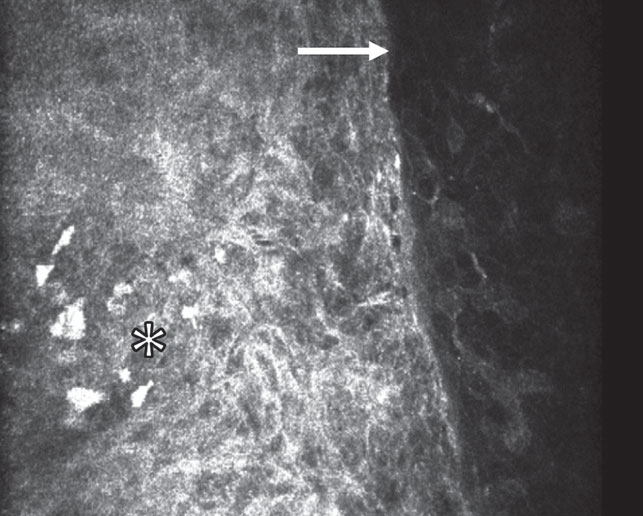 |
|
Figure 5. (A) In vivo confocal microscopy image of ocular surface squamous neoplasia. Note the sharply demarcated hyperreflective lesion with visible hyperreflective cell nuclei (asterisk). The junction between normal and abnormal tissue is abrupt (arrow). |
In-vivo confocal microscopy is an imaging tool that allows for morphological and quantitative analysis of ocular surface tissue at a microscopic and cellular level, with up to 800x magnification and impressive resolution.39 Current iterations of IVCM cite resolutions of 4 µm (axial) and 2 µm (lateral).40 Since the 1990s, IVCM has emerged as a useful diagnostic tool in the repertoire of corneal specialists.41
• Advantages. IVCM has also been used to examine cellular features of OSSN. Features include anterior and middle layers of epithelium containing hyperreflective pleomorphic squamous cells, superficial stroma laden with nuclear mitotic figures, and a clear transition between normal and neoplastic epithelium and pleomorphic cells.42 Furthermore, IVCM has been used to evaluate cellular response to topical chemotherapy in OSSN, with detection of reduced epithelial cell reflectivity, a less-defined transition between normal and abnormal tissue and fragmentation of abnormal cell clusters (Figure 5).42 IVCM has also been shown to differentiate between PAM with and without atypia. Differentiating features include a large network of dendritic cells and hyperreflective granules throughout all layers of the epithelium in PAM with atypia, and smaller dendritic cells and hyperreflective granules limited to the basal epithelium in PAM without atypia.43,44 IVCM hasn’t been shown to differentiate lymphomatous lesions from inflammatory lesions, with similar findings of highly reflective small round cells diffusely arranged in epithelium and subepithelium in both.1,43
• Disadvantages. IVCM has several factors which limit its use in the assessment of ocular surface lesions. One limitation is its small field of view, which represents only a small portion of the lesion in one plane at each point. Furthermore, the currently available software hasn’t been able to define landmarks that can aid the clinician in determining what part of the lesion is being imaged. In addition, studies have shown that both malignant and benign lesions may have cellular changes; because of this, IVCM can’t be used in isolation to diagnose a particular lesion.2,43 Cellular-level detail is also obscured in lesions with a hyperkeratinized component.45,46 Like UBM, contact with tissue is required, as is technical expertise in order to capture the images and interpret them.
• Imaging findings. A number of studies have attempted to characterize tumors of the ocular surface using IVCM. Several OSSN features have been described on IVCM. One study described dysplastic cells, with nuclear mitotic figures and nests of vortex cells in the superficial layers of the stroma.47 Another study reported hyperreflective pleomorphic cells and an absence of sub-basal corneal nerves in OSSN-involved epithelium. Finally, a third study described OSSN as having a “starry sky” appearance with enlarged, irregular and hyperchromatic nuclei with bright dots in the basal cell.42 However, benign lesions such as pterygia, pinguecula and papilloma were also found to contain similar cellular irregularities, with one case series of 60 patients demonstrating a sensitivity and specificity of 38.5 percent and 66.7 percent, respectively, when comparing OSSN to benign conjunctival lesions.48
The appearance of pigmented lesions has also been described on IVCM. Conjunctival nevi exhibited collections of uniform, medium-sized stromal cells along, with pseudocyst-like structures. PAM with atypia features large dendritic cells and hyperreflective cells throughout the epithelium, whereas PAM without atypia has smaller dendritic cells and hyperreflective cells limited to the basal epithelium. CM has large cells with prominent nuclei and nucleoli, with invasion signified by hyperreflectivity in the subepithelial layers.44 Conjunctival lymphoma features on IVCM include a normal epithelium and copious, small, tightly-packed hyperreflective cells in a cyst-like space, correlating with lymphocytes in the stromal tissue.43,49
In conclusion, the arsenal of diagnostic imaging modalities at the ocular-surface oncologist’s disposal are numerous and versatile in their strengths and weaknesses. All four have been used, in various situations, as adjuncts to the diagnosis, management and follow-up of patients with ocular surface tumors, and all can aid clinicians in examining lesions to a far greater extent than is possible with the slit lamp alone.
Correspondence: ckarp@med.miami.edu; Bascom Palmer Eye Institute, University of Miami, Miller School of Medicine, 900 NW 17th Street, Miami, FL 33136, USA
Dr. Zein is a cornea research fellow at Bascom Palmer and with the McKnight Vision Research Center. He holds a Master’s of Vision Science and Investigative Ophthalmology.
Mr. Djulbegovic is an MD candidate at the University of Miami Miller School of Medicine. He holds a MSc in Bioinformatics and Computational Biology.
Dr. Galor is a professor of ophthalmology at Bascom Palmer and a staff physician at the Miami VAMC.
Dr. Teira is a senior ophthalmic echographer in the Ocular Oncology Service at Bascom Palmer and a retina specialist.
Dr. Karp is a professor of ophthalmology, the Richard K. Forster Chair in Ophthalmology and the Dr. Ronald & Alicia Lepke Endowed Professorship in Cornea and Ocular Surface Diseases at Bascom Palmer Eye Institute, at the University of Miami Miller School of Medicine.
The authors declare that they have no conflict of interest.
1. Nanji AA, et al. Updates in ocular surface tumor diagnostics. Int Ophthalmol Clin 2017; 57:3:47-62.
2. Venkateswaran N, Sripawadkul W, Karp CL, The role of imaging technologies for ocular surface tumors. Curr Opin Ophthalmol 2021; 32:4: 369-378.
3. Ong SS, Vora GK, Gupta PK. Anterior segment imaging in ocular surface squamous neoplasia. J Ophthalmol 2016; 2016:5435092 [Epub].
4. Izatt JA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol 1994;112:12:1584-9.
5. Ang M, et al. Anterior segment optical coherence tomography. Prog Retin Eye Res 2018;66:132-156.
6. Venkateswaran N, et al. High resolution anterior segment optical coherence tomography of ocular surface lesions: A review and handbook. Expert Review of Ophthalmology 2021;16:2:81-95.
7. Huang D, et al. Optical coherence tomography. Science 1991;254:5035:1178-81.
8. Thomas BJ, et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf 2014;12:1:46-58.
9. Atallah M, et al. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. The Ocul Surf 2017;15:4:688-695.
10. Karp CL, et al. Use of high-resolution optical coherence tomography in the surgical management of ocular surface squamous neoplasia: A pilot study. Am J Ophthalmol 2019;206:17-31.
11. Yim M, et al. Ability of novice clinicians to interpret high-resolution optical coherence tomography for ocular surface lesions. Can J Ophthalmol 2018;53:2:150-154.
12. Shousha MA, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology 2013;120:5:883-91.
13. Alzahrani YA, et al. Primary acquired melanosis: Clinical, histopathologic and optical coherence tomographic correlation. Ocul Oncol Pathol 2016;2:3:123-7.
14. Nanji AA, et al. High-resolution optical coherence tomography as an adjunctive tool in the diagnosis of corneal and conjunctival pathology. Ocul Surf 2015;13:3:226-35.
15. Tanenbaum RE, et al. Classification, diagnosis, and management of conjunctival lymphoma. Eye and Vision 2019;6:1:22.
16. Venkateswaran N, et al. Optical coherence tomography for ocular surface and corneal diseases: A review. Eye Vis (Lond) 2018;5:13.
17. Kieval JZ, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology 2012;119:3:481-6.
18. Lozano García I, et al. High resolution anterior segment optical coherence tomography for differential diagnosis between corneo-conjunctival intraepithelial neoplasia and pterygium. Arch Soc Esp Oftalmol (Engl Ed) 2020;95:3:108-113.
19. Shields CL, et al. Anterior segment optical coherence tomography of conjunctival nevus. Ophthalmology 2011;118:5:915-9.
20. Koç İ, Kıratlı H. Current management of conjunctival melanoma part 1: Clinical features, diagnosis and histopathology. Turk J Ophthalmol 2020;50:5:293-303.
21. Venkateswaran N, et al. The use of high resolution anterior segment optical coherence tomography for the characterization of conjunctival lymphoma, conjunctival amyloidosis and benign reactive lymphoid hyperplasia. Eye Vis (Lond);2019:6:17.
22. de Carlo TE, et al. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 2015.1:5.
23. Pichi F, Roberts, Neri. The broad spectrum of application of optical coherence tomography angiography to the anterior segment of the eye in inflammatory conditions: A review of the literature. J Ophthalmic Inflamm Infect 2019;91:18.
24. Anvari P, et al. Artifacts in optical coherence tomography angiography. J Ophthalmic Vis Res 2021;162:271-286.
25. Siddiqui Y, Yin J. Anterior segment applications of optical coherence tomography angiography. Semin Ophthalmol 2019;34:4:264-269.
26. Lee WD, et al. Optical coherence tomography angiography for the anterior segment. Eye Vis (Lond) 2019;6:4.
27. Tan ACS, et al. An overview of the clinical applications of optical coherence tomography angiography. Eye (Lond) 2018;32:2:262-286.
28. Ang M, et al. Optical coherence tomography angiography for anterior segment vasculature imaging. Ophthalmology 2015;122:9:1740-7.
29. López-Sáez MP, et al. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol 1998;81:5:428-30.
30. Hope-Ross M, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:3:529-33.
31. Nampei K, et al. Comparison of ocular surface squamous neoplasia and pterygium using anterior segment optical coherence tomography angiography. Am J Ophthalmol Case Rep 2020; 20:100902.
32. Liu Z, et al. Role of optical coherence tomography angiography in the characterization of vascular network patterns of ocular surface squamous neoplasia. Ocul Surf 2020;18:4:926-935.
33. Brouwer NJ, et al. Anterior segment octa of melanocytic lesions of the conjunctiva and iris. Am J Ophthalmol 2021;222:137-147.
34. Martin R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian J Ophthalmol 2018;66:2:195-201.
35. Bianciotto C, et al. Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases. Ophthalmol 2011;1187:1297-302.
36. Ho VH, et al. Ultrasound biomicroscopy for estimation of tumor thickness for conjunctival melanoma. J Clin Ultrasound 2007;35:9:533-537.
37. Finger PT, et al. High-frequency ultrasonographic evaluation of conjunctival intraepithelial neoplasia and squamous cell carcinoma. Arch of Ophthalmol 2003;121:2:168-172.
38. Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol 1992;113:4:381-389.
39. Cruzat A, Qazi Y, Hamrah. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf 2017;15:1:15-47.
40. Bille JF, ed. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Cham: Springer, 2019.
41. Cavanagh HD, et al. Confocal microscopy of the living eye. CLAO J 1990;16:1:65-73.
42. Zarei-Ghanavati M, et al. Changes in in vivo confocal microscopic findings of ocular surface squamous neoplasia during treatment with topical interferon alfa-2b. Ocul Surf 2018;16:2:235-241.
43. Cinotti E, et al. Handheld reflectance confocal microscopy for the diagnosis of conjunctival tumors. Am J Ophthalmol 2015;159:2:324-33.e1.
44. Messmer EM, et al. In vivo confocal microscopy of pigmented conjunctival tumors. Graefes Arch Clin Exp Ophthalmol 2006;244:11:1437-45.
45. Parrozzani R, et al. In vivo confocal microscopy of ocular surface squamous neoplasia. Eye (Lond) 2011;25:4:455-60.
46. Xu Y, et al. The clinical value of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Eye (Lond) 2012;26:6:781-7.
47. Liang QF, et al. Histopathology manifestation and imaging characteristics of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Zhonghua Yan Ke Za Zhi 2018;54:9:652-660.
48. Nguena MB, et al. Diagnosing ocular surface squamous neoplasia in East Africa: Case-control study of clinical and in vivo confocal microscopy assessment. Ophthalmology 2014;121:2:484-91.
49. Pichierri P, et al. In vivo confocal microscopy in a patient with conjunctival lymphoma. Clin Exp Ophthalmol 2008;36:1:67-9.



