CORNEAL DYSTROPHIES ENCOMPASS A NUMBER OF DIFFERENT conditions, with a wide variety of approaches to their diagnosis and treatment. To uncover the latest developments, we asked several prominent ophthalmologists to share their current work in this area. Their comments include profiles of a new nonsurgical option for treating recurrent corneal erosions, insight into the advantages of the new "mushroom keratoplasty" technique for corneal transplant, the latest data on using mitomycin-C following laser photoablation, and a way to reduce visual aberration via diamond-burr polishing of Bowman's layer. For the latest on these topics and more, read on.
| Polishing Bowman's Layer | |
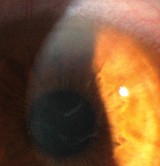
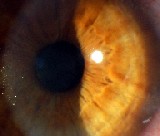
| Anterior basement membrane dystrophy (ABMD), or map-dot fingerprint dystrophy, is associated with both recurrent erosions and epithelial bumpiness. The latter can cause irregular astigmatism, glare and haloes, and generally poor vision. Christopher Rapuano, MD, co-director of the cornea service and refractive surgery departments at Wills Eye Hospital and professor of ophthalmology at Jefferson Medical College of Thomas Jefferson University in Philadelphia, has been using epithelial scraping and diamond burr polishing of Bowman's layer as a way to smooth out the cornea and reduce or eliminate the visual distortion associated with ABMD. (See pre- and postop images, right.) "Epithelial scraping and diamond burr polishing have often been used to treat recurrent erosions," says Dr. Rapuano. "But I'm not aware of anyone using this approach to treat ABMD vision problems."
Dr. Rapuano says he recently conducted a study using this approach in 13 eyes of 10 patients; mean best-corrected vision improved from about 20/50 to 20/25, and no patient lost any lines of BCVA. His results are to be published soon in the American Journal of Ophthalmology. |
Dehydrex, a nonsurgical treatment for recurrent corneal erosions, is currently in clinical trials as an orphan drug for possible approval by the U.S. Food and Drug Administration. Dehydrex is a colloidal solution, slightly hyperosmotic to the corneal stroma, that removes fluid from epithelial cells on the corneal surface. Its molecular structure can't penetrate into injured cells, so its effect is maintained even on damaged or abnormal epithelium.
Gary Foulks, MD, Arthur and Virginia Keeney Professor of Ophthalmology at the University of Louisville School of Medicine, has worked with Dehydrex for more than 20 years. "We conducted a large open-label study and two randomized, masked trials involving about 120 patients when I was at Duke University," he says. The results of his early studies were published in 1981.1
"When you have a corneal erosion, you've broken the underlying hemidesmesomes and anchoring fibrils that hold the epithelial cells in place," he explains. "If the epithelium heals over but hasn't attached to the underlying cornea, any fluid that accumulates in the basal or subbasal epithelial layer will interfere with the reformation of those attachments. At night when the eyelid is closed, the fluid can build up, lifting the epithelial cells from the
surface. In the morning, the eyelid abrades the cells and erosion results."
Dr. Foulks says that Dehydrex works well to help control recurrent corneal erosions, regardless of the cause. "I haven't had to refer a patient for PTK in six or seven years," he says. However, he notes that the drug doesn't stop the erosions immediately. "I typically prescribe one drop five times a day in the affected eye for three to five months," he says. "The patient usually notices symptomatic relief fairly quickly, but less-frequent and shorter-duration episodes of erosion may continue for a while.
"I wait until the patient has been without symptoms for a month, and I haven't seen anything at the slit lamp suggesting residual basal cell edema or microcysts for a month," he explains. "At that point I continue the therapy for an additional two months, because studies have suggested that it takes at least eight weeks for cellular reattachments to form when the corneal epithelium is healing."
Dr. Foulks says symptoms can return if the erosions are due to dystrophy rather than trauma, but he'd wait until they reappear before trying to treat them again. However, some patients don't want to stop the treatment because the erosions are so disruptive. "One patient used the drop for four years without any problem," he adds. Dr. Foulks says that he has also had success using Dehydrex to treat dry-eye syndrome.
John Szabocsik, PhD, a long-time expert in regulatory issues and managing clinical trials, is helping Helles Laboratory Inc. conduct the clinical trial of Dehydrex. "To finish the current trial," he says, "we need a few more patients, but the inclusion criteria make it hard to find qualified participants. We can include additional investigational sites, so if you have patients who might be interested in participating, please contact me at jszaboc@sbcglobal.net."
Mushroom Keratoplasty
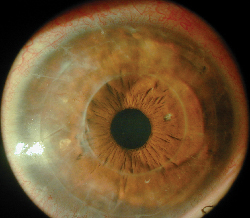
Massimo Busin, MD, clinical associate professor at Louisiana State University Health Science Center in New Orleans, has recently developed a novel technique for transplanting corneal tissue that combines a large lamellar graft with a smaller penetrating central graft, creating a mushroom-shaped area of donor tissue. The procedure works well for patients with full-thickness opacities but a healthy endothelium, such as those with stromal dystrophies.
|
Massimo Busin, MD |
| Following "mushroom keratoplasty," two segments of donor cornea can be seen. |
"The advantage of a large graft on the front is that large grafts are known to have better refractive results," he says. "But if you do a full-thickness large graft, the risk of rejection is higher. By combining the anterior large graft with the posterior small graft, you retain the advantages of both procedures and eliminate the disadvantages."
Dr. Busin says that he begins the procedure by removing the mushroom "head and stem" sections of the patient's cornea. "First, I make a partial thickness 9-mm incision, about 200-µm deep, using a trephine," he says. "I do the dissection of the lamellae by hand because the resulting surface will be outside of the visual axis. This creates a slightly rougher interface, which allows more wound healing to take place, creating a stronger bond between the tissues. Then I punch out the central part of the stromal and endothelial tissue completely, leaving a central hole of 5 or 6 mm to match the posterior graft."
Next, Dr. Busin splits the donor cornea into anterior and posterior lamellae using a microkeratome. "I make the top slice of the donor cornea section as wide as possible," he explains. "Then I take it and punch it to size using the 9-mm trephine. I use another trephine to punch out a 5- or 6-mm central graft from the posterior lamella."
Dr. Busin places the "mushroom stem" in the central hole of the recipient's stromal bed with no sutures. He then places the superficial lamellar flap in the corresponding hole in the recipient's cornea, fixing it with a running 10-0 nylon suture. After surgery, the patient uses antibiotic and steroid drops for two weeks, tapering over a period of about three months; then the sutures can be removed.
Dr. Busin says he's done the procedure about 40 times, including one lattice dystrophy. In a case study reported in the July, 2005 issue of the American Journal of Ophthalmology, the patient's best corrected visual acuity improved from 20/60 preop to 20/20 at six months postoperatively.2
Dr. Busin points out another advantage of this procedure: The small diameter of the posterior section of the graft may prevent the need for further surgery even if the immune system rejects the donor endothelial cells. "The normal endothelial cell count is about 3,000 cells per square millimeter," he explains. "If you remove the central 5 mm of endothelial lamellae, you're actually removing less than 20 percent of the endothelium. These cells don't replicate, but they move along the basement membrane from areas of higher density to areas of lower density. If the donor endothelial cells die, the recipient's healthy cells may spread over the graft and revitalize it. The density would still be sufficient to keep the cornea clear."
Dr. Busin hopes to give a presentation outlining the one-year results of mushroom keratoplasty on 18 patients at the next American Academy of Ophthalmology meeting.
Mitomycin-C After PTK
Eric Donnenfeld, MD, co-chairman of cornea and external disease at Manhattan Eye, Ear and Throat Hospital and associate professor of ophthalmology at New York University Medical Center, has been experimenting with the use of mitomycin-C following the use of excimer laser photoablation to treat corneal dystrophies.
"Treating dystrophy with phototherapeutic keratectomy can eliminate the need for more invasive surgery such
as a corneal transplant," notes Dr. Donnenfeld. "However, dystrophy often recurs after PTK. Also, the deep ablation can cause fibrosis and haze. We've found that the use of adjunctive mitomycin-C can significantly reduce the risk of these problems."
Dr. Donnenfeld has used adjunctive mitomycin-C on two cases of Reis-Bückler's dystrophy and three cases of granular dystrophy.3 He currently has a year and a half of follow-up on the Reis-Bückler's cases. "We haven't seen any recurrence of dystrophy," he notes. "More importantly, we haven't seen any fibrosis. The corneas are more optically clear; the patient's best-corrected visual acuity is better, and we don't have to worry about haze and related complications. This has been true for all of these cases."
Dr. Donnenfeld says that to his knowledge use of mitomycin-C has been safe in corneal surgery. "I think that's because we're applying it to the terminally differentiated central cornea, rather than the limbus, where the primary stem cells are found," he says. "Also, we've cut our mitomycin-C use from two minutes to 15 seconds, and still get the same results. We haven't seen any complications. These are patients who otherwise would need a corneal transplant, so I think the risk-reward ratio here is extremely high."
Dr. Donnenfeld isn't the only surgeon who has noted the potential of mitomycin-C under these conditions. In 2002, one study reported a successful PTK retreatment of anterior basement membrane dystrophy and Salzmann's nodular dystrophy with a single application of mitomycin-C; no complications or recurrences occurred within six months of the procedure.4
Also, in a presentation at the May 2005 meeting of the Association for Research in Vision and Ophthalmology, Christopher Rapuano, MD, and Brandon Ayres, MD, presented results of a retrospective chart review of 13 eyes of 11 patients treated with PTK and mitomycin-C for anterior corneal disease. They found that the average time epithelial defects took to heal was three days; there was no evidence of corneal or scleral melting, and minimal postoperative haze. One patient with Salzmann's nodule and one with Reis-Bückler's dystrophy had mild recurrences in the periphery, outside the area treated with PTK.
Deep lamellar endothelial keratoplasty (DLEK), a procedure developed by Mark A. Terry, MD, director of corneal services at Devers Eye Institute in Portland, Ore., continues to generate interest as an alternative to penetrating keratoplasty in dystrophies that affect only the posterior surface of the cornea, such as Fuchs' dystrophy. In this procedure, the surgeon creates a limbal scleral tunnel incision and removes the diseased endothelial tissue, replacing it with similar, but healthy, tissue from a donor eye. The procedure has several advantages over penetrating keratoplasty, including faster recovery of vision, no suturing, no severing of nerves in the anterior cornea, and possibly a reduced rate of rejection.
| Transplants and Cataract Surgery |
| Walter J. Stark, MD, distinguished professor of ophthalmology and director of the Stark-Mosher Center for Cataract and Corneal Diseases of The Wilmer Eye Institute in Baltimore, may have inspired a revision in the American Academy of Ophthalmology's Preferred Practice Pattern for corneal dystrophy. Until recently, the guidelines recommended that when patients with Fuchs' corneal dystrophy underwent cataract surgery, simultaneous corneal transplant should be considered if the patient's cornea was thicker than 600 µm. The premise was that a thick cornea would not have an endothelium healthy enough to withstand the surgery without undergoing corneal decompensation. However, Dr. Stark had been able to perform cataract surgery on Fuchs' patients and produce a very clear cornea without corneal decompensation, even when corneas were up to 640-µm thick. "I perform very careful cataract surgery, using a viscoelastic such as Viscoat to protect the endothelium," he explains. Because of his success with these patients, he was surprised when a colleague who had undergone training at Moorfield's Hospital in London reported that the 600-µm recommendation was being strictly adhered to. Dr. Stark felt that this meant that many patients were being subjected to unnecessary risks, complications, expense and a lengthy recovery. Dr. Stark asked one of his residents to conduct a review of his Fuchs' dystrophy cataract cases performed between October 1990 and May 2002. "Out of about 50 cases with a corneal thickness of 600 µm or more, our success rate was greater than 90 percent at an average two-year follow-up," he says. "That means that if we had adhered to the old recommendations, 90 percent of those patients would have had unnecessary corneal transplants." In fact, even his patients with a corneal thickness of 640 µm or greater did well; 83 percent didn't need a corneal transplant during the follow-up period. Their postoperative BCVAs ranged from 20/20 to 20/200, averaging 20/50. Dr. Stark emphasizes that it's important to consider each case individually. "Some patients have a corneal thickness less than 640 µm, but have obvious epithelial edema," he explains. "That means their corneas have already decompensated, and they need a transplant. But the majority of people with corneal thickness up to 640 µm don't have corneal edema, and they'll withstand careful cataract surgery." Even if corneal thickness is greater than 640 µm, which tends to reduce quality of vision even when the cornea is clear, Dr. Stark notes that some elderly or one-eyed individuals might be better off with reduced quality of vision than having a corneal transplant. At this year's American Society of Cataract and Refractive Surgery meeting, Jeremy Seitman, MD, the resident who conducted the review, presented a paper with the results. The paper suggested revising the recommendation for corneal transplant during cataract surgery to "greater than 640 µm," with an addendum that avoiding a corneal transplant might also be reasonable above 640 µm if visual acuity of less that 20/20 is acceptable. Dr. Stark recently noted that the guideline recommendation has now been raised to 640 µm. "The change in this indication will reduce the health-care costs for those people and the waiting time for visual recovery," notes Dr. Stark. "It's significant, and it changes the way we practice medicine for the better." |
• Study results. "A report appearing in the September 2005 issue of Ophthalmology reviews the six-month results of our first 100 DLEK patients," says Dr. Terry, "in terms of visual acuity, astigmatism, endothelial survival and complication rates. The data show that DLEK produces better results than penetrating keratoplasty, including not adding to the eye's astigmatism. At six months the average visual acuity was between 20/40 and 20/50 in this elderly population."
Another study focusing on DLEK complications, including dislocation rate, aggravated glaucoma and rejection, has been accepted for publication by Cornea.
• Cutting the donor tissue with a microkeratome. The original DLEK procedure uses a manual dissection instrument co-designed by Dr. Terry to remove tissue from both donor and recipient. Now several investigators are using a microkeratome to cut off the top part of the donor cornea, attempting to create a smoother interface. "It seems logical that a smoother interface on the donor would produce better visual acuity," notes Dr. Terry, "although this hasn't been demonstrated in a scientific study yet. Smoothness may also impact healing, but at this point we don't have enough evidence to draw a conclusion."
•Using the femtosecond laser. "We're also investigating using the femtosecond laser on both patient and donor," says Dr. Terry. "Currently, the laser creates a nice, smooth interface in the anterior stroma for LASIK surgery, but a much rougher interface deep in the stroma, as was shown in an eye-bank study recently published in Cornea.5 However, work is being done to change the way the laser applanating cone is configured, to try to create a smoother interface."
Stripping Descemet's Membrane
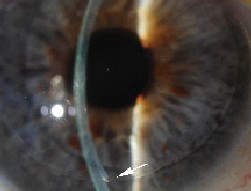
Francis W. Price Jr., MD, director of the Price Vision Group in Indianapolis, has been doing corneal work for 25 years and is a pioneer in the use of the Descemet's stripping and endothelial keratoplasty technique (DSEK). DSEK is similar to DLEK, except that the only thing that's removed from the patient's eye is Descemet's membrane and the endothelium. A thicker slice of donor tissue is still used as the implant.
"I've been using the Descemet's stripping technique for four years and have done about 350 cases," he says. "The majority of our patients see 20/40 by three months. That's a big improvement over PK."
Dr. Price used DLEK for his first 100 cases. "The original thought was, if you're going to put a piece of donor cornea in, you should take a similar piece out of the patient's eye. So, we'd remove the back 20 or 30 percent of the cornea.
|
Francis W. Price, MD |
| The edge of the donor graft is visible six months after DSEK. |
Marianne Price, PhD, director of research for the Cornea Research Foundation of America, notes that removing Descemet's membrane is a bit like peeling off a layer of Saran Wrap; taking only the membrane from the donor cornea and trying to implant it would be extremely difficult. "We take the membrane with part of the stroma from the donor eye, which gives the piece of tissue the consistency of a soft taco, so you can fold it in half to insert through a 5-mm scleral tunnel incision into the eye." An air bubble is used to hold the donor tissue in place.
One advantage of this technique is that it can be used when a PK graft fails. "If the surface of the cornea is still healthy, we can use the Descemet's stripping technique to replace the endothelium instead of redoing the entire transplant. We've done 10 of these cases so far, and the patients are really happy. Some of them were one-eyed patients who might have gone a year or two without really good vision if we'd done another full transplant."
| Genotyping and Diagnosis |
| Research into the genetic factors behind corneal dystrophy continues at multiple laboratories across the country. Richard W. Yee, MD, a clinician and scientist at the University of Texas Health Science Center at Houston and a professor at the university, runs a genetics lab funded by the National Institutes of Health. His group is particularly interested in anterior dystrophies such as Thiel-Behnke. "One of the uses of DNA testing is to determine a specific diagnosis," he explains. "Dystrophies in this part of the cornea, including Reis-Bücklers', lattice type 1, and Meesman's, are very distinct, but they may look the same when seen by an ophthalmologist at the slit lamp. Today, many of these anterior stromal dystrophies can be treated with a laser, so you don't get any histology to help with a diagnosis. The only way to know which disease you're looking at is to get DNA from the patient's blood and do genotyping." Asked about the advantages of identifying a specific dystrophy, Dr. Yee admits that it's still too early to manipulate the genes and prevent further symptoms. "However, having the diagnosis and knowing which category to put the patient in allows you to define the disease, what course will it follow, and what you and the patient can expect," he says. "You can take preventive steps; if you know that a child has the gene, you can create an optimal environment so the surface of the cornea doesn't break down as fast. "Knowing the exact nature of the disease also makes it possible to provide valuable family counseling," he says. "Parents can find out whether their children are likely to develop the problem; if so, they can take preventive steps as needed. If not, everyone can save themselves some worry." Currently, Dr. Yee and his colleagues are creating a corneal registry. "People send blood to us all the time," he says. "Someday when treatment modalities become available, we'll already have people identified and categorized by their mutation so we can effectively treat them." Dr. Yee encourages doctors to submit patients with interesting dystrophies for inclusion in the registry. "We offer free genotyping for families with dystrophies. When a referring doctor believes a patient has a dystrophy and wants to confirm the mutation, he sends us a blood sample and a photograph of the disease. We then determine which category it fits in. We've been providing this service for the past few years, with partial funding from NIH." If you'd like to inquire about free corneal dystrophy genetic testing, you can contact Dr. Yee at richard.w.yee@uth.tmc.edu, or call (713) 306-3051 and ask for Richard W. Yee. For more information, visit http://uth.tmc.edu/ophthalmology/corneal_gen/index.htm on the Web. |
Dr. Terry has also tried DSEK, and expresses concern about tissue dislocation. "Reports suggest that the dislocation rate is much higher with DSEK than with the DLEK manual technique," he notes. "Also, I haven't seen any data indicating that DSEK produces better vision than the DLEK procedure. I'm concerned that some surgeons will choose an operation with a higher complication rate simply because it's easier to do."
Dr. Price agrees that dislocation is an issue. "In the first 10 cases we did the detachment rate was about 50 percent," he says. "To manage this we've modified the way we put in the tissue and hold it in position, and we've decreased the rate to somewhere between 3 and 6 percent.
"It's also important to realize that it's easy to reattach the tissue if it does become detached," adds Ms. Price. "You just put in another air bubble the next day."
Dr. Price notes that these are difficult procedures, and recommends that people take a course before they try them. Dr Price offers a course in DSEK, as do Gerrit R. J. Melles, MD, PhD, (the surgeon who developed the DSEK technique) in Holland, and Dr. Terry.
An article scheduled for the July/August issue of the Journal of Cataract & Refractive Surgery will cover the visual results of Dr. Price's first 50 DSEK cases.
1. Foulks GN. Treatment of recurrent corneal erosion and corneal edema with topical colloidal osmotic solution. Ophthalmology 1981;88:801-803.
2. Busin M, Arffa RC. Microkeratome-assisted Mushroom Keratoplasty With Minimal Endothelial Replacement. Am J Ophthalmol. 2005;140:1:138-40.
3. Miller A, Solomon R, Bloom A, Palmer C, Perry HD, Donnenfeld ED. Prevention of recurrent Reis-Bucklers dystrophy following excimer laser phototherapeutic keratectomy with topical mitomycin C. Cornea. 2004;23:7:732-5
4. Marcon AS, Rapuano CJ. Excimer laser phototherapeutic keratectomy retreatment of anterior basement membrane dystrophy and Salzmann's nodular degeneration with topical mitomycin C. Cornea 2002;21:828-30.
5. Terry MA, Ousley PJ, Will B. A practical femtosecond laser procedure for DLEK endothelial transplantation: cadaver eye histology and topography. Cornea. 2005;24:4:453-9.