As a chronic disease, glaucoma marches steadily toward irreversible vision loss. Treatment focuses on preventing and slowing the development and progression of optic nerve damage with IOP-lowering measures such as topical and systemic drugs, selective laser trabeculectomy and incisional surgery.1
Trabeculectomy turned 50 in 2018, and today it remains the gold standard for the surgical management of glaucoma. With half a century behind it, trabeculectomy has a long record of published results and well-refined techniques that have made it one of the most effective, cheapest and simplest options for glaucoma surgeries.2 But despite being the most used surgical method for managing glaucoma, trabeculectomy still fails and often yields inconsistent results,2 such as postoperative IOPs that are either too low or too high, and complications such as hyphema, decompression retinopathy and choroidal detachment.3 As a result, alternative procedures with better safety outcomes and lower risk profiles have been sought.
Most experts agree that having MIGS as an additional, less-invasive surgical option for their patients is valuable in the overall arc of glaucoma care. In the interest of increasing safety and reducing the number of surgical interventions needed to manage glaucoma, however, you may wonder if doing MIGS procedures earlier on is worth it if some patients still wind up needing additional, more invasive procedures down the road. In this article, surgeons share their thoughts on MIGS’ impact on the arc of glaucoma care and the role these procedures play in staving off blindness.
What MIGS Offers
MIGS has been touted as a potential alternative to conventional incisional surgeries, as well as to medication and laser treatments, but surgeons agree that it’s not a panacea. So what can you expect from MIGS? When asked whether or not MIGS obviates the need for more traditional surgery, Michele C. Lim, MD, professor, vice chair and medical director of ophthalmology at UC Davis, replied that “MIGS doesn’t supplant some of the more traditional surgeries.”
Rather than serve as a replacement for traditional trabeculectomy or tube shunts, most surgeons say that MIGS works best as an additional option for glaucoma treatment or as a delaying procedure. “MIGS temporarily shifts and delays the need for trabs and tubes,” points out Sean Ianchulev, MD, MPH, professor of ophthalmology at the New York Eye and Ear Infirmary. “Patients are given another temporizing surgical treatment alternative that fills the gap between drops and conventional glaucoma surgery.” Brian Francis, MD, MS, associate professor of ophthalmology at the Doheny Eye Institute, Keck School of Medicine, USC, agrees and notes that additionally, “MIGS is a safer surgical alternative to trabs and tubes when used properly.”
In an editorial published in Ophthalmology in 2015, Ike Ahmed, MD, notes that “MIGS devices are often used earlier in the glaucoma treatment algorithm...A common misperception of MIGS is that it needs to be compared with the gold standard of mitomycin-C trabeculectomy to show its effectiveness. This inappropriate interpretation is based on the idea that MIGS procedures are designed to replace conventional filtering surgery. In fact, MIGS devices are designed to address the treatment gap that exists between medical therapy and more aggressive traditional surgical options.”5
Philip Bloom, MB ChB, FRCS(Ed), FRCOphth, consultant ophthalmic surgeon at Imperial College Healthcare NHS Trust in London, says that MIGS is a completely new step in the surgical paradigm. “With MIGS, a proportion of patients avoid the need for more invasive surgery, but MIGS are rarely in competition with trabeculectomy, which is, in my opinion, the best single option for when you need very low pressures and for patients with advanced disease. If we get patients early enough, we’ll often do a MIGS device either to prevent their being on too many drops or to even avoid starting drops in the first place.
“It’s entirely possible that, as they become more effective, MIGS may take on a role more comparable to trabeculectomy, but at the moment they’re not as effective,” he says. “They fit earlier into the treatment paradigm than trabs or tubes.”
 |
MIGS Has Its Place
MIGS’ success depends in large part on proper patient selection, Dr. Francis says. “MIGS is particularly suited to mild-to-moderate glaucoma cases, especially in patients whose pathology is in the trabecular meshwork. This includes pseudoexfoliation glaucoma, pigmentary glaucoma and steroid-induced glaucoma. On the other hand, MIGS is unsuitable for a patient with very advanced glaucoma who needs target pressures of 10 to 12 mmHg, for example.
“The biggest advantage of MIGS in general is its enhanced safety versus traditional filtering surgery and the fact that it gives patients more options,” he continues. “You’re giving surgical options to patients who either wouldn’t have been surgical candidates before because they had mild-to-moderate glaucoma or to those patients for whom it’s unsafe to do filtration surgery.”
“Recovery time after a MIGS procedure is usually shorter than after traditional surgery, depending on which MIGS you’re talking about,” Dr. Lim notes. “Additionally, in a MIGS surgery, you’re moving less tissue in the eye.” This may be especially important for older patients; because surgical procedures rely a good deal on the quality of the surgical tissue, older patients are at a higher risk for complications.4 Dr. Lim also notes that moving less tissue in the eye will open up more options if you need to do another surgery down the road.
However, for more severe cases of glaucoma, “MIGS doesn’t routinely get eye pressures into the low teens,” says James C. Tsai, MD, MBA, president of the New York Eye and Ear Infirmary of Mount Sinai and system chair of ophthalmology, Mount Sinai Health System. “I think it’s good we have MIGS,” he says. “I think the reason I don’t do MIGS is that I’m not sure how much it helps for the types of patients that I see, who tend to have moderate to severe glaucoma, such as very complicated glaucomas, normal-tension glaucomas or glaucomas where they’ve already been on multiple medications. In those cases, patients usually don’t have very high pressures. Their pressures tend to be in the mid to high teens or maybe low 20s. Many of my patients need pressures in the low teens or high single digits for the glaucoma progression to halt. If you look at the prospective MIGS studies, eye pressures tend to end up in the mid to high teens.”6
“You want to save the more invasive surgeries for when the glaucoma is more severe,” Dr. Tsai continues. “It’s not very fair to ask that MIGS have comparable IOP-lowering efficacy to a trab or tube in a moderate-to-severe case.” Most MIGS clinical trials have been restricted to mild-to-moderate cases of glaucoma, and as a result, there’s little data on MIGS’ efficacy for moderate-to-severe glaucoma.1
A 2018 literature review of MIGS outcomes found that the MIGS devices used in the analyzed studies were typically associated with higher postoperative IOPs, compared to various forms of trabeculectomy, which usually result in IOPs between 11 and 13 mmHg. The review included the outcomes of nine randomized clinical trials (seven iStents, one Hydrus and one of the now de-funct CyPass stents), seven non-randomized clinical trials (three iStents, three CyPass and one Hydrus) and 23 economic studies. The authors also found that MIGS devices may result in increased hypotony rates or bleb needling in devices placed subconjunctivally. It was unclear whether the cost of MIGS is outweighed by the cost-savings of medication reduction or less need for further intervention.6
MIGS and Cataracts
For most MIGS procedures to be reimbursed by insurance, they must be done in conjunction with cataract surgery. (Allergan’s Xen gel stent is the only FDA-approved exception to this rule.) Dr. Tsai says this is one of MIGS’ major drawbacks. “Essentially, the patient has to need/have cataract surgery for you to really consider MIGS,” he says.
Furthermore, cataract surgery isn’t benign, Dr. Tsai adds. “A lot of our patients are myopic, and myopia is a risk factor for glaucoma,” he says. “The risk of retinal detachment in myopes is likely 3 to 5 percent over the patient’s lifetime, approximately a three to five times higher risk than cataract surgery in non-myopes. Moreover, some patients’ cataracts aren’t severe enough to warrant cataract surgery. Making a patient pseudophakic isn’t well-received by all patients, especially those who still have the ability to accommodate for near work.
“If a patient has a high risk for infection, suprachoroidal hemorrhage or has a difficult time coming back for regular follow-up visits, then maybe a cataract-MIGS procedure is a procedure that you as a glaucoma surgeon should perform,” Dr. Tsai says. “If you can combine the cataract surgery with another procedure to get the pressure down another 1 to 2 mmHg more than the pressure lowering with cataract surgery alone, that might be sufficient. And maybe in this particular patient, the effect with MIGS is greater—say 3 to 4 mm more—than with cataract surgery alone. As a result, that patient might be able to get off her medications or reduce the number of medications she’s on.
“However, you’re already going to get a pressure reduction with cataract surgery alone,” he adds. “That’s been clearly demonstrated.7 But oftentimes, it’s hard to say how much of the pressure reduction was from the MIGS procedure or simply due to the patient responding very well to the IOP-lowering effects of small-incision cataract surgery. Based on what I’ve seen in the literature and with my own patients who’ve had previous MIGS procedures, the results aren’t significantly better with than cataract surgery alone.”8
With outcomes similar to cataract surgery, Dr. Francis says MIGS is sometimes overused. “I’ve seen some patients with intraocular hypertension have cataract surgery and a MIGS procedure. For someone who has ocular hypertension, the pressure will lower with just the cataract surgery anyway.”14
A 2015 Cochrane review of nine randomized controlled trials compared combined surgery to cataract surgery alone in eyes with co-existing cataract and glaucoma. The nine trials reviewed included 655 participants (657 eyes) with follow-up periods ranging from 12 to 30 months. Glaucoma surgeries included three studies with trabeculectomy, three studies with iStent implants, one study with trabeculotomy and two studies with trabecular aspiration. All of the studies showed a statistically significant greater decrease in mean IOP postoperatively in the combined surgery compared to cataract alone. However, with few studies reviewed, the authors concluded that there’s only low-quality evidence that combined cataract and glaucoma surgery may result in better IOP control at one year compared to cataract surgery alone.8
Dr. Lim points out that FDA-approved MIGS devices have shown an advantage in IOP-lowering compared to cataract surgery alone.9-11 “The crux of FDA approval is showing superiority with the device,” she says. “Those studies are designed to randomize patients to receive cataract surgery alone or combined surgery. The FDA looks at the device study endpoints, such as a 20-percent reduction from baseline IOP, and those devices that achieve a higher proportion of patients reaching that endpoint, versus cataract surgery alone, receive approval.”
HORIZON Study
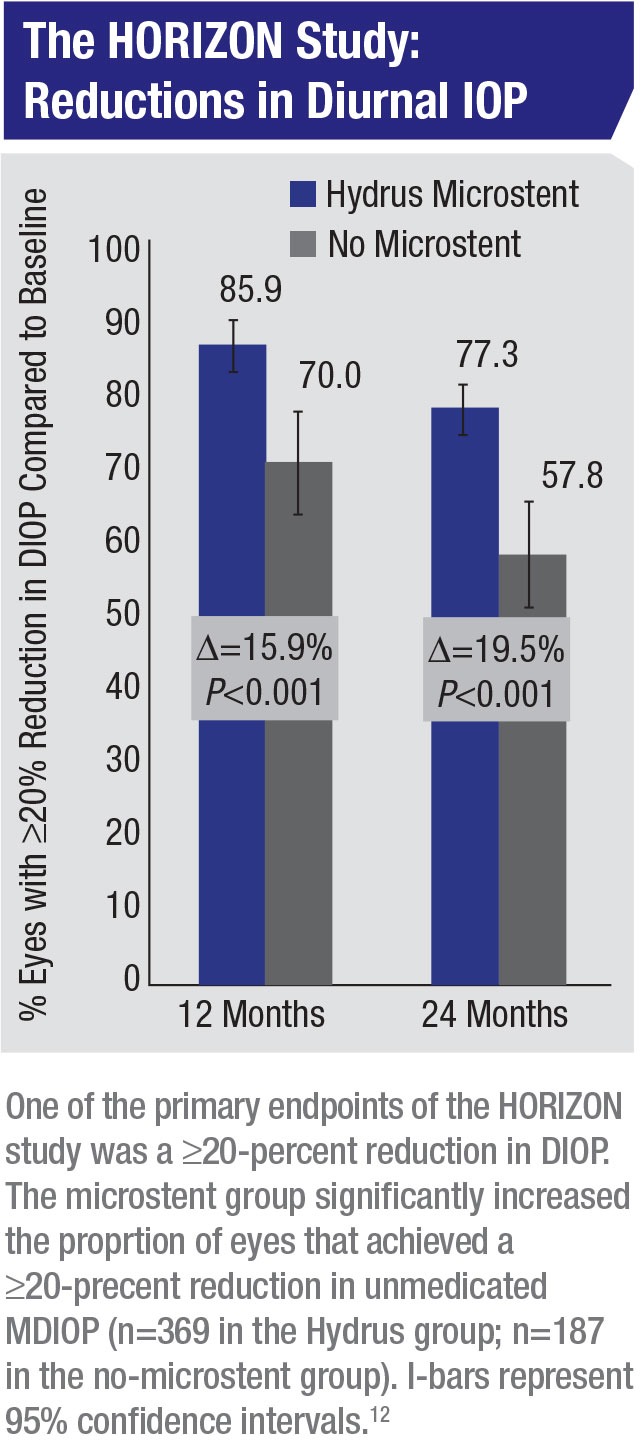
Recently, a large, global, prospective, randomized, controlled trial was conducted for the Hydrus Microstent to test its safety and efficacy in lowering IOP in glaucoma patients undergoing cataract surgery.11 It should be noted that this study included only mild to moderate cases of glaucoma. The three-year results found that 73 percent of Hydrus patients remained medication-free, compared to 48 percent of cataract-only patients. Among the patients who entered the study on one medication (like about half of glaucoma patients in the United States), 81 percent remained medication-free, compared to 48 percent in the cataract-only arm.11 The study found that the combined MIGS procedure had an overall safety profile similar to that of cataract alone, including endothelial cell density stability from year two to three and no significant changes between the Hydrus and cataract-only groups. Of note, only 0.6 percent of Hydrus patients went on to receive subsequent invasive surgery to control their glaucoma, which the company says was a first.
 |
Though the HORIZON study demonstrated a reduction in the need for subsequent invasive surgery, some of the statistically significant findings are less impactful for cases of glaucoma that are more serious than just mild-to-moderate, further supporting many experts’ view that, while MIGS is effective in some cases and a valuable option, it’s not yet ready to replace trabeculectomy.
Dr. Tsai explains that “in the HORIZON study, the investigators found that cataract surgery with the Hydrus device lowered the pressure an average of 7.6 ±4.1 mmHg while the cataract surgery with no microstent lowered the pressure an average of 5.3 ±3.9 mmHg. So the difference was 2.3 mmHg (95 percent CI; p<0.001),” he says. “The mean number of medications was reduced from 1.7 ±0.9 at baseline to 0.3 ±0.8 at 24 months in the Hydrus group and from 1.7 ±0.9 to 0.7 ±0.9 in the no-microstent group, a difference of -0.4 medications (p<0.001)—about half a medication.12 Is a 2.3-mmHg difference significant for your patient? I’d say it depends whether the IOP drop is maintained long-term. Certainly, though, a 2-mmHg difference between patients who received cataract alone and those who received the combined MIGS procedure is promising. However, we don’t know the efficacy of these procedures in moderate to severe glaucoma.”
Dr. Tsai adds that “the U.S. cohort in the HORIZON trial concluded at 24 months follow-up that the implantation of the Schlemm’s canal microstent significantly reduced medications, compared to phaco-only in patients with mild to moderately severe glaucoma. The mean change in medications was -1.2 ±0.9 in the microstent group and -0.8 ±1.1 in the phaco-only group (p<0.001), so that’s still only a 0.4-medication difference.”13
What’s After MIGS?
“Managing glaucoma therapy is a bit like being a billiards player,” Dr. Lim says. “For every treatment you offer the patient, you have to think three steps into the future. You have to think: If I offer a patient a certain surgery and if it fails, I need to have another plan. What am I going to do next?”
She recommends using MIGS for cases where the need for additional surgery is highly likely. “I think MIGS can definitely fill a role in earlier stages of disease for controlling the pressures, as well as for some people who have more severe disease where you know you’ll have to ramp up the type of surgery you offer them in the future,” she says.
Prof. Bloom says that waiting until the glaucoma is more severe to undergo incisional surgery may increase the risk of complications and failure. “A study from 1989 on the benefit of early trabeculectomy showed that eyes which lost the most visual field were those with the least field loss at diagnosis,” he says. “This paradox was attributed to a prolonged attempt at medical control in these eyes because they were thought to have a lower risk of visual field deterioration.22 If you put off necessary surgery, patients get worse.”
That’s one reason why MIGS is an important addition to the surgeon’s toolkit as an earlier intervention. “MIGS has very few downsides apart from the cost,” Prof. Bloom notes. “Even if MIGS doesn’t work out very well, you haven’t lost much—you haven’t lost a ‘bite of the surgical cherry.’ You can still proceed with a trabeculectomy or a tube later on.
“The results can be somewhat variable,” he continues, “but if you do get a good response, then in that patient you might be able to avoid doing a trab. If someone has an average or suboptimal response, you can still go on to a trabeculectomy, and the fact that you’ve previously done MIGS hasn’t lost you anything. As long as you haven’t lost too much time trying to assess whether it works or not, then MIGS is a very low-risk option, really.”
While MIGS procedures are sometimes followed by a trab or tube, Prof. Bloom cautions that there are still risks with having prior conjunctival-incisional glaucoma surgeries. “The risk is scarring,” he says. “The efficacy of trabeculectomy relies on relative lack of scarring because the aqueous comes out of a biological valve that’s formed on the surface of the eye by the operation. The more operations you do that involve the conjunctiva, the greater the risk of failure. So for example, if someone’s had a Xen implant or a PreserFlo (formerly known as the InnFocus Microshunt) that impinges upon the conjunctiva, the chances of a trabeculectomy working after one of those procedures may be reduced. However, if patients have an iStent or a Hydrus or one of those other devices that don’t touch the conjunctiva, then theoretically the chance of a trabeculectomy working after a procedure like that is every bit as high as if they hadn’t had it. MIGS procedures don’t compromise future conjunctival surgery in the way that other conjunctival surgeries do.”
Limited Lifetime
“If we follow the outcome of MIGS surgeries long enough, we may see MIGS’ effects wearing off and patients needing to go back on medications and/or have additional operations,” Dr. Tsai says. “Many of the prospective studies for MIGS look promising, but after a longer period of follow up, we might start to see the eye pressures beginning to creep back up. That’s why a robust follow-up period is needed to assess the impact and efficacy of MIGS. In my opinion, 24 months is the minimum needed. Anything shorter won’t be conclusive enough. That said, the HORIZON trial was helpful since it reported both 24-month and three-year data.”
A study evaluating the long-term efficacy and safety of combined MIGS and cataract surgery with the iStent implant for coexistent cataract and glaucoma found that combined surgery seems to be an effective and safe avenue for treatment.15 The mean follow-up period was 53.68 ±9.26 months and the data showed a 16.33-percent decrease in IOP (19.42 ±1.89 mmHg to 16.26 ±4.23 mmHg [p=0.002] at the end of follow-up). One author of the study is a consultant for Glaukos.
Since glaucoma is a chronic, incurable set of diseases, effectiveness over time is an important variable to consider when selecting treatments, experts say. “You should try to delay the more aggressive surgeries until you really need them,” advises Dr. Francis. “A patient controlled for five years with a MIGS procedure who then goes on to receive a trabeculectomy can probably get through her lifetime without significant vision loss. Whereas, if you do the trabeculectomy right off the bat and it fails after five years, your options are more limited at that point. Of course sometimes patients require a more aggressive surgery early on, and if they need it, they need it.”
Using MIGS to delay a potential need for more invasive surgery is a good method for prolonging the effectiveness of glaucoma treatments. Lifetime efficacy is one goal of treatment, though the meaning of “lifetime” has shifted over the years as average life expectancy increases. Between 1959 and 2016, the U.S. life expectancy increased from 69.9 years to 78.9 years.20 Though overall U.S. life expectancy has declined since 2014 due to midlife mortality caused by drug overdoses, alcohol abuse, suicide and various organ system diseases20 (78.84 years in 2014 to 78.69 years today),21 glaucoma patients diagnosed in their late 60s and early 70s can still expect to live for at least another decade. In the United States, the life expectancy for men and women at age 71, respectively, is 13.72 years and 15.82 years.18
On presentation, the average age was 71.1 years in a 2007 mortality study conducted in the United Kingdom that followed 57 patients diagnosed with chronic open-angle glaucoma. The study found that, after 10 years, two-thirds of the patients survived, and that for most older, white patients, preventing a visual handicap is achievable 10 years after diagnosis.16 At the 15-year follow-up period for the same cohort, the researchers found that 44 percent of the patients had died.17 Medium-term results over five-year periods demonstrate a continuing lowering in IOP and medications,19 but “with longer follow-up we would no doubt observe increasing surgical failure,” Leon Au, MD, says in a debate piece originally presented at the Royal College of Ophthalmologists Congress in 2017.19 “But the benefit these procedures offer in that all-important five-to-seven-year period for this group of elderly patients is invaluable,” he concludes.
Future Developments
The consensus among experts seems to point to MIGS as a valuable addition to the glaucoma treatment arc, but not a cure-all, and certainly not a replacement for traditional filtering surgery in severe cases of glaucoma. Currently, glaucoma treatments’ safety and efficacy profiles are at odds with each other. “MIGS offers the patient a pressure lowering treatment with a high safety profile,” Dr. Lim says. “But with a high safety profile, comes MIGS’ limited efficacy.” Trabeculectomy and tube shunts have more efficacy, but at the expense of safety.
Prof. Bloom says future iterations of MIGS designs will likely provide an equal safety profile with greater efficacy than current models. “We’re still very early in the development cycle,” he says. “We’re faced with first-, second- and third-generation devices, whereas, for example, intraocular implants are perhaps on their fifteenth or twentieth generation and becoming ever more refined. When MIGS become more effective, but maintain their high safety profile, then at that point they may start to challenge traditional surgeries in terms of efficacy, whilst beating them hands down in terms of safety.”
Dr. Ianchulev says he envisions a positive future for MIGS. “It’s bright and glorious,” he says. “It’s opening up a new category which will be here to stay.” REVIEW
Dr. Tsai is a consultant for Eyenovia, ReNetX and Smartlens. Dr. Francis discloses relationships with Neomedix, MST, BVI, Endo Optiks, Glaukos, Allergan and New World Medical. Dr. Lim is an investigator for Santen and a speaker for Alcon. Dr. Ianchulev discloses financial relationships with Eyenovia. Prof. Bloom has no paid consultancy relationships to disclose, but notes that he has previously been paid to participate in advisory boards for Glaukos and to lecture for EndoOptiks.
1. Bar-David L, Blumenthal EZ. Evolution of glaucoma surgery in the last 25 years. Rambam Maimonides Med J 2018;9:3:e0024.
2. Bhartiya S, Dhingra D, Shaarawy T. Revisiting results of conventional surgery: Trabeculectomy, glaucoma drainage devices, and deep sclerectomy in the era of MIGS (editorial). J Curr Glaucoma Pract 2019;13:2:45-49.
3. Ramona B, Pop M, Paul-Eduard S, et al. Intraoperative and postoperative complications in trabeculectomy, clinical study. Rom J Ophthalmol 2015;59:4:243-247.
4. Raczyńska D, Glasner L, Serkies-Minuth E, et al. Eye surgery in the elderly. Clin Interv Aging 2016;11:407-414.
5. Ahmed IK. MIGS and the FDA: What’s in a Name? (editorial). Ophthalmol 2015;122:9:1737-39.
6. Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther 2018;7:49-73.
7. Berdhal JP. Cataract surgery to lower intraocular pressure. Middle East Afr J Ophthalmol 2009;16:3:119-122.
8. Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database Sys Rev 2015;7.
9. Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: A meta-analysis. PLoS One 2015;10:7.
10. Akil H, Chopra V, Francis B, et al. Short-term clinical results of ab interno trabeculotomy using the Trabectome with or without cataract surgery for open-angle glaucoma patients of high intraocular pressure. J Ophthalmol 2017;1-9.
11. Ivantis announces groundbreaking 3 year results from FDA clinical trial; first device in minimally invasive glaucoma surgical (MIGS) category to demonstrate significant long term reduction of severe major surgeries for glaucoma patients (press release). Ivantis 2019. Accessed Jan 29, 2020.
12. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: The HORIZON Study. Ophthalmol 2019;126:1:29-37.
13. Jones J, Koch DD, Vold S, et al. Results from the United States cohort of the HORIZON trial of a primary Schlemm canal microstent to reduce intraocular pressure in primary open-angle glaucoma. J Cataract Refract Surg 2019;45:9:1305-1315.
14. Mansberger S, Gordon M, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: The ocular hypertension treatment study. Ophthalmol 2012;119:9:1826-31.
15. Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: A long-term study. Br J Ophthalmol 2012;96:5:645-649.
16. Sharma T, Salmon JF. Ten-year outcomes in newly diagnosed glaucoma patients: Mortality and visual function. Br J Ophthalmol 2007;91:10:1282-4.
17. Shahid H, Salmon JF. Fifteen-year mortality rate and visual outcome in newly diagnosed chronic open-angle glaucoma. Br J Ophthalmol 2013;97:2:235-6.
18. Actuarial Life Table: Period Life Table, 2016. Social Security Administration. ssa.gov/oact/STATS/table4c6.html. Accessed Jan 29, 2020.
19. Bloom P, Au L. “Minimally invasive glaucoma surgery (MIGS) is a poor substitute for trabeculectomy”—The great debate. Ophthalmol Ther 2018;7:203-210.
20. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA 2019;322:20:1996-2016.
21. United States Life Expectancy. World Bank via Google Search Results graphics. worldbank.org. Accessed Jan 29 2020.
22. Jay JL, Allan D. The benefit of early trabeculectomy versus conventional management in primary open angle glaucoma relative to severity of disease. Eye 1989;3:528-535.



