Michael D. Ober, MD
Christina M. Klais, MD
Emmett T. Cunningham Jr., MD, PhD, MPH
New York City
Macular edema represents the pathologic accumulation of extracellular fluid within the retina, primarily in the outer plexiform and inner nuclear layers, as a nonspecific response to a breakdown in the blood-retinal barriers. ME is a frequent cause of vision loss in patients with diabetes mellitus, retinal venous occlusion, uveitis, and following intraocular surgery. It occurs less frequently in the setting of vitreoretinal traction, choroidal neovascularization and a number of other conditions. Many strategies have been employed to manage ME with varying success. This article reviews the available treatment options for this common condition.
 |
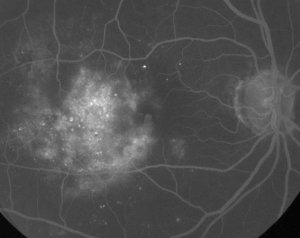 |
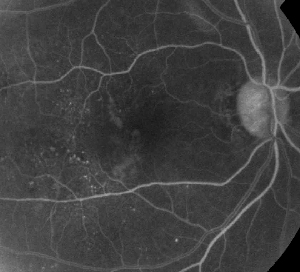
| Figure 1. A. Early phase fluorescein angiography of a patient with non-proliferative diabetic retinopathy. Microaneurysms are seen most prominently temporal to the fovea in addition to fluorescein leakage in the foveal avascular zone. | B. Late-phase FA of the same patient showing diffuse leakage temporal to and within the foveal avascular zone corresponding to diabetic macular edema. |
Diagnosis
The clinical diagnosis of ME is best made using a contact lens and stereoscopic slit-lamp fundus biomicroscopy. ME typically manifests as an irregular elevation within the retina, often adjacent to intraretinal lipid, microaneurysms, and/or hemorrhages in cases secondary to diabetes mellitus, vascular occlusion, or ischemia. Intraretinal fluid may also accumulate in cystic spaces localized to the parafoveal retina with or without adjacent vascular abnormalities. This cystoid macular edema (CME) results most commonly from inflammation, localized traction, or following surgery.
Fluorescein angiography is an essential tool in the diagnosis of ME. In the normal eye, fluorescein is prevented from passing into the retina by the blood-retinal barriers. In ME, however, fluorescein molecules leave the intravascular space to enter the retina. The effected sites show hyperfluorescence in early to mid frames that increases in area and intensity in later frames (See Figure 1). FA not only highlights edema for easy visualization and treatment localization, but also creates a permanent record for future comparison. A four-grade quantitative scale was developed for ME, wherein grade 0 is no perifoveal hyperfluorescence, grade 1 is incomplete perifoveal hyperfluorescence, grade 2 is mild 360-degree hyperfluorescence, grade 3 is moderate 360-degree hyperfluorescence with the hyperfluorescent area being approximately 1 disc diameter across, and grade 4 is severe 360-degree hyperfluorescence with the hyperfluorescent area being approximately 1.5 disc diameter across.1,2 While FA is a sensitive means of identifying the presence of ME, it provides relatively little information regarding the anatomical distribution of the fluid; i.e., diffuse vs. cystic vs. subretinal, and the severity of the leak over time. FA provides no quantitative information regarding retinal thickening. It is not surprising, therefore, that overall this two-dimensional FA-based grading system correlates poorly with vision.3,4
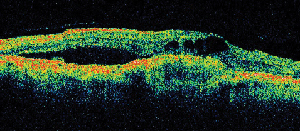
Optical coherence tomography (OCT III, Carl Zeiss) is a non-contact, noninvasive imaging technique that provides a useful adjunct in diagnosing ME. It directs a beam of near infra-red light (830-nm) perpendicular to the surface of the retina and analyzes the properties of the reflections. In 1.5 seconds, it produces a single linear high resolution cross-sectional image. These images can display and even measure the thickened, cystic retina found in edematous areas. It is also useful in visualizing the properties of the vitreoretinal interface and effectively demonstrates when vitreous traction plays a role in the formation of ME (See Figure 2).
One study used OCT to examine 84 eyes with ME secondary to uveitis, which provided the added benefit of revealing or confirming the presence of epiretinal membranes and serous retinal detachment in 41 and 20 percent of their cases, respectively.5 The study found a moderate correlation between retinal thickness and decreasing visual acuity, although the degree of correlation has varied across studies with other investigators reported weak,6 moderate,7 and strong8,9,10 correlations using varying statistical methods in diverse patient populations, including patients with diabetic retinopathy, uveitis and CME.
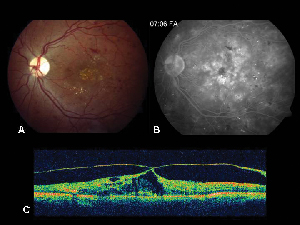 |
| Figure 2. A. Color fundus photograph of the left eye of a patient with non-proliferative diabetic retinopathy and lipid exudation in and around the fovea. B. Late-phase FA reveals macular edema in the central macula. C. Optical coherence tomography demonstrates the abnormal vitreoretinal interface as well as macular edema. |
Another group reported that OCT was as effective as FA in detecting ME and superior to FA in describing the axial distribution of fluid.6 OCT also has the ability to identify some patients with ME not visualized on FA, as in certain cases of chronic CME where the sort of active leakage best visualized with FA is minimal or even absent, or when fundus examination is compromised by the presence of media opacities, such as in patients with asteroid hyalosis.11
The retinal thickness analyzer (RTA, Talia Technology, Israel) is a similar non-contact imaging technique that allows for quantification of retinal thickness. It produces 16 parallel cross-sectional scans over a 3x3 square mm area of retina by analyzing reflections from an obliquely directed pulse of green light (540 nm) delivered over 0.3 seconds. Both OCT and RTA have shown excellent precision in their individual measurements of retinal thickness, and a direct comparisons of the technologies has demonstrated a statistically significant correlation between their measurements. While RTA has the advantage of rapid acquisition with perhaps fewer artifacts, it appears to be less effective at measuring retinal thickness than OCT in the presence of media opacities.12
Another study compared foveal thickness with RTA and OCT in 30 healthy eyes. The mean foveal thickness in the normal eyes was measured at 181 µm and 153 µm for RTA and OCT, respectively. The authors concluded that RTA occasionally produces false high values, and thus has reduced reliability compared to OCT.13 In contrast, an analysis of patients with mild non-proliferative diabetic retinopathy found that RTA was more sensitive than OCT in identifying areas of retinal thickening during the initial stages of diabetic ME.14
 |
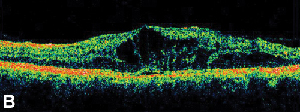 |
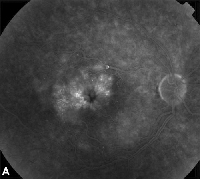
| Figure 3. A. Late phase fluorescein angiogram of a patient with cystoid macular edema. | B. Optical coherence tomography showing the large cystic spaces at the fovea. |
Treatments —Medical
Topical non-steroidal anti-inflammatory medications are the most common treatment for ME following cataract surgery (See Figure 3). These agents are directed at decreasing intraocular prostaglandin levels, which have been implicated in the pathogenesis of ME. Double-masked, randomized, active and placebo-controlled studies including patients undergoing cataract surgery have reported anti-inflammatory effects from topically applied 1% indomethacin, 0.03% flurbiprofen, 0.5% ketorolac, and 0.1% diclofenac ophthalmic preparations.15,16 Diclofenac 0.1% and ketorolac 0.5% ophthalmic solutions, however, are the only topically applied NSAIDs specifically approved by the Food and Drug Administration for this indication. Therapy combining a topical corticosteroid and NSAID drops has been found to have greater efficacy in treating ME than either medication alone.16 Although not FDA-approved, topical NSAIDs are often used prior to cataract surgery to prevent postoperative ME.
Oral acetazolamide is occasionally used in the treatment of ME secondary to inflammatory conditions and retinitis pigmentosa, particularly when topical NSAIDs and corticosteroids fail. Several prospective, masked, crossover studies comparing acetazolamide and placebo in patients with ME due to a variety of causes. A five-cycle crossover study in 41 patients found a reproducible response, characterized as either partial or complete resolution of ME, in more than half of the patients with inherited or inflammatory retinal disease, but no response from those with primary retinal vascular diseases.17 A 500-mg/day or oral acetazolamide was found to be more effective than 250-mg/day in treating ME in patients with ME secondary to RP.18 This study observed improvement in 10 of 12 treated patients.
Another group concluded that patients under 55-years-old with ME attributed to chronic iridocyclitis were more like to respond to 500 mg acetazolamide b.i.d. than older patients.19 A recent study has suggested that acetazolamide may also be effective for the treatment of diabetes induced ME as well.20
Corticosteroids are potent anti-inflammatory agents that are used frequently in the treatment of ME. They have multiple mechanisms of action, including stabilization of the blood-retinal barrier and inhibition of pro-inflammatory mediators. Delivery modes include topical, periocular injection, intravitreal injection, and both oral and intravenous administration. While oral and IV corticosteroids certainly reach therapeutic levels within the vitreous, they expose patients to the additional risk of systemic complications, and are therefore usually reserved for patients with sight-threatening uveitis in the setting of systemic disease. Topical corticosteroid drops are at the other end of the safety spectrum, but their ability to reach the posterior segment is limited.
Sub-Tenon's injections offer an alternative to deliver relatively high doses of corticosteroids to the eye with lower risks of systemic complications.21 Though there have been no randomized, controlled trials, sub-Tenon's corticosteroid injection has been used effectively in treating macular edema for many years. The most common technique uses a short 25-ga. needle placed through the superotemporal bulbar conjunctiva into the sub-Tenon's space while the patient looks inferonasally. The needle is advanced posteriorly along the globe using a sweeping side-to-side motion to prevent inadvertent globe penetration, until the hub reaches the conjunctival entry site, when the medication is delivered. One report included 20 consecutive patients with intermediate uveitis associated with vision loss who were treated with sub-Tenon's injection of 40 mg triamcinolone acetonide.22 Although not all patients demonstrated ME on FA, 67 percent improved by two lines of vision following one treatment. Risks of this procedure include persistently elevated intraocular pressure, cataract, ptosis and intraocular penetration among others.
Recently, the use of intravitreal injection of triamcinolone acetonide (Kenalog, 4.0 mg) has increased due to its potent ability to ameliorate refractory ME secondary to diabetes mellitus (See Figure 4), retinal venous occlusions, inflammation, and other idiopathic causes.23-26 Preliminary studies show dramatic reduction in retinal thickening, decreased fluorescein leakage, and visual improvement, which may be marked in some patients. Although the effect is temporary and typically lasts for three to six months or less, the ME usually responds to re-injection. The use of intravitreal corticosteroids is associated with a 30- to 40-percent risk of persistently elevated intraocular pressure and an approximate 10-percent risk of cataract requiring surgery, however. The National Eye Institute is currently enrolling patients for SCORE, the standard care vs. corticosteroid for retinal vein occlusion study, which compares intravitreal injections of triamcinolone (1- and 4-mg doses) with standard care (observation and/or grid laser treatment) in patients with ME secondary to vein occlusion. The study will follow a total of 1,260 patients and continue treatment for 36 months.
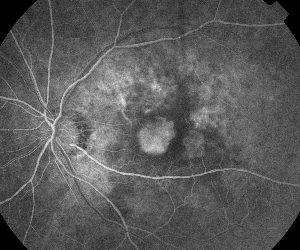
ME also occurs in with age-related macular degeneration (See Figure 5). Intravitreal injection of triamcinolone acetonide is currently under investigation for combined use with photodynamic therapy for the treatment of neovascular AMD (Visudyne with intravitreal Triamcinolone Acetonide, VisTA). One study emphasizes the importance of intravitreal triamcinolone preceding the application of PDT in patients with macular edema and CNV.27 The authors theorize that verteporfin may leak into cystic intraretinal spaces leading to photoreceptor damage of adjacent, normal retina once the drug is photoactivated, while prior resolution of retinal edema may prevent this complication. Pre-administration of corticosteroid may also serve to blunt any PDT-induced elevation in intraocular VEGF levels.
Intravitreal injections themselves are associated with small, but definite risks of serious, potentially blinding side effects including infectious endophthalmitis, retinal detachment, hemorrhage, ocular hypertension, cataract and hypotony.28 Recently published guidelines for intravitreal injections attempt to establish a best-practices approach for this increasingly used technique. The consensus panel's recommendations addressed pre-injection considerations including antibiotics, glaucoma evaluation, glove use, and treatment of pre-existing eyelid abnormalities, as well as the importance of avoiding excessive lid manipulation before and during the procedure. The recommended peri-injection regimen included the use of topical and/or subconjunctival anesthetic, topical povidone iodine, and an eyelid speculum. The authors stressed the importance of monitoring IOP and direct fundus visualization following the injection to verify perfusion of the optic nerve, intravitreal location of triamcinolone, and the absence of injection-associated hemorrhage or retinal detachment. They also stressed the importance of patient education vis-à-vis early symptoms of potential complications and thorough follow up.29
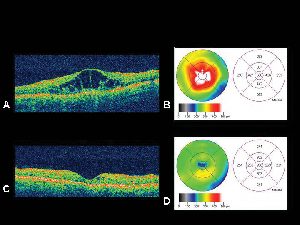 |
| Figure 4. A. Optical coherence tomography image of an eye with diabetic macular edema with corresponding retinal thickness mapB. generated by the OCT. C. OCT of the same patient one month following intravitreal triamcinolone acetonide injection withcorresponding retinal thickness map. D. Resolution of macular edema. Visual acuity improved from 20/200 to 20/80 following treatment. |
Several trials are investigating alternative long-term corticosteroid delivery devices for use inside the eye. One study involves the surgical placement of a fluocinolone acetonide pellet embedded on a plastic strut with controlled release of steroids over three years. Known as the Envision intravitreal implant, by Bausch & Lomb and Control Delivery Systems, it is placed through the pars plana and sutured to the sclera. A Phase-II/III randomized, masked study compared Envision TD implant in 80 patients randomized to 0.5- vs. 2.0-mg fluocinolone acetonide vs. standard of care consisting of laser photocoagulation or observation. After six months, a statistically significant reduction in ME and severity of diabetic retinopathy was seen with the 0.5-mg implant compared to standard of care. In addition, there were no differences in the incidence of serious adverse effects. The 2.0-mg arm of the study was stopped early due to the results observed in a second study in which no advantage over 0.5 mg was noted. A second study enrolled 278 patients with non-infectious posterior uveitis randomized to receive either a 0.59-mg or 2.1-mg Retisert implant the affected eye or, in bilateral cases, in the more severely afflicted eye. After two doses and 34 weeks, there was a significantly lower recurrence rate in eyes with the implant (10 vs. 55.7 percent, p<0.0001) with a decrease in use of systemic corticosteroid/immunosuppressive therapy (59.0 percent at baseline vs. 13.7 percent at 34 weeks) as well as sub-Tenon's and topical steroid use. There was a significant improvement in visual acuity (p<0.05). The most common adverse events included cataract progression and increased intraocular pressure which required a filtering procedure in 8.6 percent. (Jaffe G. Invest Ophthalmol Vis Sci. 2004; 44 ARVO E-Abstract #3369.)
Another trial involves Allergan's Posurdex, a bioerodable dexamethasone pellet injected into the vitreous space that releases medication over 50 to 160 days. Results of a Phase-II trial revealed that both a 350-µg and 700-µg pellet significantly improved the percentage of patients with two lines or greater improvement in vision (27.2 percent and 35.7 percent 350 µg and 700 µg, respectively) as well as a three line or greater improvement in vision (13 percent and 19.4 percent, for 350 µg and 700 µg, respectively) at 180 days compared to placebo. Thus far, there has been no increased incidence of cataract reported, though IOP increases were seen in about15 percent of patients.
Treatments —Laser
Most recently, Eyetech's and Pfizer's Macugen was studied in a randomized, double-masked, multicenter, dose-ranging, controlled Phase II trial of 172 patients with diabetic ME. The study investigated three doses (0.3 mg, 1.0 mg, 3.0 mg) verses sham injections given every six weeks for three injections. While prior focal/grid laser investigators were asked only to enroll patient in whom they felt comfortable deferring focal/grid laser for at least 12 weeks. Additional injections and/or focal/grid photocoagulation were given at the investigator's discretion from week 18 to 30. Final assessments were conducted at week 36, six weeks after the last planned injection. Overall, subjects assigned to receive Macugen had better vision outcomes, were more likely to show reduction in central retinal thickness, and were deemed less likely to need additional laser therapy as compared to sham patients.31 A confirmatory Phase III study is currently planned.
Focal/grid laser photocoagulation remains the standard of care for the treatment of diabetic ME. The Early Treatment Diabetic Retinopathy Study showed that patients treated with grid laser had a 50-percent reduction in moderate visual loss, defined as a doubling of the visual angle or a three-line decrease in vision, when compared with observation.30 Although the exact mechanism by which laser decreases ME is unknown, it is believed to promote the formation of tight junctions between RPE cells as well as reduce oxygen demand from photoreceptors and increase oxygen perfusion from the choroid.31 The EDTRS identified patients eligible for focal laser photocoagulation as having clinically significant ME. This was defined as meeting one of the following three criteria: 1) retinal thickening located within 500 µm of the fovea; 2) hard exudates less than 500 µm from the fovea associated with adjacent retinal thickening; or 3) an area of edema 1 disc diameter or greater, any part of which located less than 1 disc diameter from the fovea. The EDTRS did not, however, distinguish between focal ME, which corresponds to local thickening of retina adjacent to microaneurysms, and diffuse ME, which refers to a generalized thickening of the posterior pole. Anecdotal evidence shows that focal diabetic ME responds well to focal/grid laser while the diffuse variety more frequently fails laser treatment and requires alternative management.
 |
 |
| Figure 5. A. Early fluorescein angiogram showing classic subfoveal neovascularization. | B. Corresponding optical coherence tomography reveals areas of subretinal and intraretinal fluid accumulation as well as demonstrating the neovascularization (arrow). |
Treatments —Surgery
The first group that reported the benefits of vitrectomy and posterior hyaloid separation in patients with diabetic ME suggested that a subgroup of patients exists in whom vitreous traction and shallow macular detachments contribute to retinal thickening.32 This has subsequently been confirmed with OCT, leading to refinement of the indications for this technique.33,34 The three largest series35,36,37 of patients undergoing vitrectomy for diabetic ME unresponsive to less invasive treatments analyzed 59, 58 and 65 patients, respectively. They reported 47 percent, 53 percent and 45 percent, respectively, of their patients improved in vision by two lines or greater. The last of these reported serious postoperative complications developed in a minority of patients including retinal detachment (1.5 percent), rubeosis iridis (4.6 percent) epiretinal membrane (13.8 percent), recurrent vitreous hemorrhage(1.5 percent), and foveal hard exudate deposits (4.6 percent) while the complications revealed the second group included epiretinal membranes in 10.2 percent and cataracts in 63.2 percent of phakic eyes. Several other case series have shown similar results, however, all were nonrandomized, without placebo control, and using different inclusion and exclusion criteria. Further, surgical techniques have also differed, leaving the exact indications for vitrectomy in patients with CME open to interpretation.
ME remains a major cause of visual loss despite the variety of available treatments. Laser photocoagulation remains an integral part of the management of ME due to diabetes, ischemia and vascular occlusions. Topical NSAIDs and corticosteroids are currently the primary method to control postoperative ME, while acetazolamide remains an effective means to treat in selected patients with ME secondary to uveitis and retinitis pigmentosa. The role of intravitreal corticosteroids in ME therapy is expanding, but remains limited by side effects and duration of effect. Refinements in surgical techniques will continue to add a new dimension to ME unresponsive to less invasive treatment while advances in pharmacotherapy and ocular drug delivery promise to play a role in the prevention and management of all causes and types of ME.
Dr. Ober is a fellow in vitreoretinal surgery at the Edward S. Harkness Eye Institute at Columbia University College of Physicians and Surgeons, and the LuEsther T. Mertz Retinal Research Center at Manhattan Eye, Ear, and Throat Hospital. Contact him at 210 East 64th St., 8th Fl, New York, NY 10021; e-mail: obermike@aol.com; or (212) 605 3777 or fax (212) 605 3795.
Dr. Klais is a retina fellow at the LuEsther T. Mertz Retinal Research Center. Contact her at the same address, phone or fax numbers, or by e-mail at cmcKlais@cswebmail.com.
Dr. Cunningham is a clinical professor of ophthalmology and director of the Uveitis Service at New York University, School of Medicine. He is also an employee of Eyetech Pharmaceuticals Inc.Contact him at Vitreous-Retina-Macula Consultants of New York, 460 Park Ave., New York, N.Y. 10022, by e-mail emmett_cunningham@yahoo.com, or by phone/fax at (212) 861 9797.
1. Spaide RF, Yannuzzi LA, Sisco LJ. Chronic cystoid macular edema and predictors of visual acuity. Ophthalmic Surg 1993;24:262-267.
2. Yannuzzi LA. A perspective on the treatment of aphakic cystoid macular edema. Surv Ophthalmol 1984;28:540-553
3. Chang A, Spaide RF, Yannuzzi LA. Post-surgical cystoid macular edema. In: Guyer DR, Yannuzzi LA, Chang S, et al. Retina, Vitreous, Macula. Saunders. Philadelphia. 1999;1:239-255.
4. Nussenblatt RB, Kaufman SC, Palestine AG, et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology 1987;94:1134-9.
5. Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology 2004;111:946-53.
6. Antcliff RJ, Stanford MR, Chauhan DS, et al. Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology 2000; 107:593-9.
7. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 1999;127:688-93.
8. Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol 1995;113:1019-29.
9. Nussenblatt RB, Kaufman SC, Palestine AG, et al. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology 1987;94:1134-9.
10. Ozdek SC, Erdinc MA, Gurelik G, et al. Optical coherence tomographic assessment of diabetic macular edema: comparison with fluorescein angiographic and clinical findings. Ophthalmologica 2005;219:86-92.
11. Browning DJ, Fraser CM. Optical coherence tomography to detect macular edema in the presence of asteroid hyalosis. Am J Ophthalmol 2004;137:959-961.
12. Polito A, Shah SM, Haller JA et al. Comparison between retinal thickness analyzer and optical coherence tomography for assessment of foveal thickness in eyes with macular disease. Am J Ophthalmol 2002;134:240-51.
13. Neubauer AS, Priglinger S, Ullrich S, et al. Comparison of foveal thickness measured with the retinal thickness analyzer and optical coherence tomography. Retina 2001;21:596-601.
14. Pires I, Bernardes RC, Lobo CL, Soares MA, Cunha-Vaz JG. Retinal thickness in eyes with mild nonproliferative retinopathy in patients with type 2 diabetes mellitus: comparison of measurements obtained by retinal thickness analysis and optical coherence tomography. Arch Ophthalmol 2002 Oct;120(10):1301-6.
15. Flach AJ. Cyclo-oxygenase inhibitors in ophthalmology. Surv Ophthalmol 1992;36:259-284.
16. Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107:2034-8.
17. Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol 1988;106:1190-5.
18. Fishman GA, Gilbert LD, Fiscella RG, Kimura AE, Jampol LM. Acetazolamide for treatment of chronic macular edema in retinitis pigmentosa. Arch Ophthalmol 1989;107:1445-52.
19. Farber MD, Lam S, Tessler HH, Jennings TJ, Cross A, Rusin MM. Reduction of macular oedema by acetazolamide in patients with chronic iridocyclitis: a randomised prospective crossover study. Br J Ophthalmol 1994;78:4-7.
20. Giusti C, Forte R, Vingolo EM, Gargiulo P. Is acetazolamide effective in the treatment of diabetic macular edema? A pilot study. Int Ophthalmol 2001;24:79-88.
21. Jennings T, Rusin MM, Tessler HH, Cunha-Vaz JG. Posterior sub-Tenon's injections of corticosteroids in uveitis patients with cystoid macular edema. Jpn J Ophthalmol 1988;32:385-91.
22. Helm CJ, Holland GN. The effects of posterior subtenon injection of triamcinolone acetonide in patients with intermediate uveitis. Am J Ophthalmol 1995;120:55-64.
23. Benhamou N, Massin P, Haouchine B, Audren F, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol 2003;135:246-9.
24. Jonas JB, Kreissig I, Degenring RF. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2002;240:782-3.
25. 25. Martidis A, Duker JS, Greenberg PB, Rogers AH, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology 2002; 109: 920-7.
26. 26. Scott IU, Flynn HW Jr., Rosenfeld PJ. Intravitreal triamcinolone acetonide for idiopathic cystoid macular edema. Am J Ophthalmol 2003;136:737-9.
27. 27. Freund KB, Klais CM, Eandi CM, et al. Sequenced Combined Intravitreal Triamcinolone and Indocyanine Green Angiography Guided Photodynamic Therapy for Retinal Angiomatous Proliferation. Arch Ophthalmol, In Press.
28. 28. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreal injection: a comprehensive review. Retina. 2004 Oct;24(5):676-98.
29. 29. Aiello LP, Brucker AJ, Chang S, et al. Evolving guidelines for intravitreal injections. Retina 2004 Oct;24(5 Suppl):S3-19.
30. .G.Jaffe, Fluocinolone Acetonide Uveitis Study Group. Fluocinolone Acetonide Intravitreal Implant for Uveitis Affecting the Posterior Segment of the Eye. ARVO 2004, poster #3369.
31. The Macugen Diabetic Retinopathy Study Group. A phase II randomized, double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology, In press.
32. 30. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985;103:1796-806.
33. Christoforidis JB, D'Amico DJ. Surgical and other treatments of diabetic macular edema: an update. Int Ophthalmol Clin 2004;44:139-60.
34. Lewis H, Abrams GW, Blumenkranz MS, Campo RV. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology 1992;99:753-9.
35. Kaiser PK, Riemann CD, Sears JE, Lewis H. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol 2001;131:44-9.
36. Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol 2001;131:123-5.
37. Pendergast SD. Vitrectomy for diabetic macular edema associated with a taut premacular posterior hyaloid. Curr Opin Ophthalmol 1998;9:71-5.
38. Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol 1996;122:258-60.
39. Yamamoto T, Hitani K, Tsukahara I, Yamamoto S, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol 2003;135:14-9.



