Workup, Diagnosis and Treatment
The differential diagnosis for a severely swollen eyelid with associated conjunctival injection includes a range of etiologies, including preseptal or orbital cellulitis, orbital abscess, necrotizing fasciitis, neoplastic processes, orbital pseudotumor, trauma, carotid-cavernous fistula and thyroid ophthalmopathy. In combination with high intraocular pressure, trauma complicated by an orbital compartment syndrome arises as an urgent consideration; however, inflammatory and infectious etiologies may also present in this fashion. Given the patient’s history of sinus disease and fever, and the absence of trauma, infectious etiologies such as orbital cellulitis and/or abscess were deemed most likely.
Immediate attention was drawn to the high IOP and the lateral canthotomy was urgently
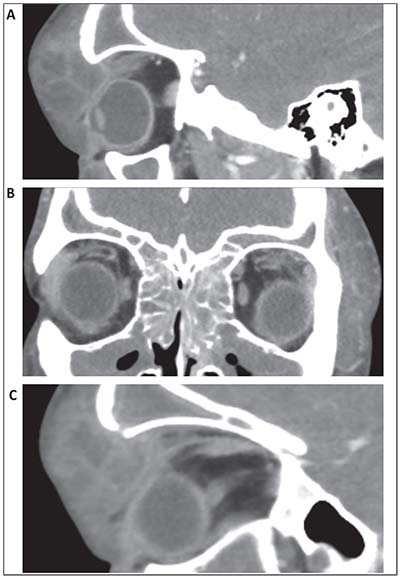 |
| Figure 2. (A) Sagittal CT image on initial presentation showing a large multi-loculated abscess with direct compression of the orbit. (B) Coronal CT image showing diffuse sinusitis of the maxillary, ethmoidal and frontal sinuses. (C) Sagittal CT image showing the frontal sinus openly-communicating with one of the lobules of the orbital abscess. |
completed with the addition of an inferior cantholysis. After the canthotomy the appearance of the eye remained unchanged and the intraocular pressure was measured at 38 mmHg with a visual acuity of 20/400. Medical therapy was instituted with three rounds of timolol-dorzolamide and brimonidine drops every 15 minutes, and an intravenous infusion of 100 g of mannitol. One hour after the medications were finished the intraocular pressure was found to be 30 mmHg.
Ancillary imaging was obtained with a CT scan of the orbits with contrast that showed a large multi-loculated abscess of the left upper lid tracking posteriorly into the orbit, disrupting normal anatomy and deforming the superior aspect of the globe (Figure 2A). Extensive opacification of bilateral maxillary, ethmoid and frontal sinuses was also seen (Figure 2B). The frontal sinus openly communicated with one of the lobules of the superior orbital abscess (Figure 2C). The patient was taken urgently to the operating room where a left superior orbitotomy with irrigation and drainage was performed. Twenty milliliters of pus was drained from the area, and a Penrose drain was placed. The Otolaryngology Service then performed functional endoscopic sinus surgery with drainage of the bilateral ethmoid, maxillary, sphenoid and frontal sinuses.
Postoperatively, the patient was treated with intravenous cefepime, metronidazole and vancomycin. Examination of the affected eye after surgery showed visual acuity of 20/200 and an intraocular pressure of 26 mmHg. By postoperative day two the patient had a visual acuity of 20/40, IOP of 23 mmHg and improved lid swelling. On day three, cultures grew Streptococcus anginosis, and the patient was switched from cefepime and vancomycin to intravenous ceftriaxone. On postoperative day five the patient continued to show significant improvement in swelling, and his visual acuity was 20/20 with an IOP of 16 mmHg. The patient was subsequently discharged on oral amoxicillin/clavulanate with close outpatient follow-up.
Discussion
Orbital cellulitis is an infectious process involving orbital tissue posterior to the orbital septum. It’s more common in the pediatric population, with an estimated incidence of 1.6 per 100,000 children versus 0.1 per 100,000 adults.1 The most common risk factor for orbital cellulitis is paranasal sinusitis, with the ethmoid sinus most frequently involved.2 Direct extension of the infection is likely facilitated by a number of orbital anatomical factors, including the thin medial orbital wall, lack of lymphatics, valveless veins and multiple foramina.3 Other predisposing associations include upper respiratory infection, trauma, surgery and orbital foreign body.2
In our particular case, orbital cellulitis presented as an uncommon manifestation of Pott’s puffy tumor—a rare complication of frontal sinusitis where frontal bone osteomyelitis occurs with associated subperiosteal abscess. First described by Sir Percivall Pott in 1768 as a result of trauma,4 PPT is now more typically associated with infectious etiologies and can extend through bone to involve the skin, brain or orbit. Classically, PPT presents with localized tender forehead swelling, but may also include headache, orbital symptoms, fever, vomiting and purulent rhinorrhea depending on the involved anatomy.5,6 Orbital involvement has been described in 33 to 45 percent of PPT cases.6,7
Close attention to potential complications of orbital infection is essential, as these can be severe, with vision- and life-threatening consequences. Extension of an orbital infection can lead to optic neuropathy, endophthalmitis, meningitis and central nervous system involvement. PPT is especially worrisome, as intracranial involvement is a relatively common and dangerous complication.5,8,9 Thrombophlebitis of orbital veins or mechanical compression of the central retinal artery can also cause severe ocular ischemic damage. As in our patient, abscesses can occur in orbital infections, and studies have shown abscesses to be more common in adults than children.2,10
The infection may also cause external pressure on the eye, and our case demonstrates the importance of recognizing the underlying mechanics of this pressure. While a classical orbital compartment syndrome involves a buildup of pressure behind the eye that may be relieved by anterior release with a lateral canthotomy and cantholysis, pressure from an anterior etiology pushing back on the globe, as in our case, may not respond to this approach. In such cases, it’s most beneficial to directly address the etiology of anterior pressure with urgent surgical intervention.
Diagnostic imaging may be critical in orbital cellulitis and CT is considered the modality of choice, given its superior bony imaging, speed and availability. Imaging is indicated in all cases of periorbital inflammation where proptosis, ophthalmoplegia, decreased visual acuity, concern for foreign body, neurological signs or lack of improvement over 24 hours with treatment are present.2,11 In addition to identifying or ruling out other etiologies of orbital inflammation, imaging may provide information regarding the degree of extension of the infection, including intracranial or cavernous sinus involvement; identify subperiosteal or orbital abscesses; assess for concurrent sinusitis and/or identify foreign bodies in the orbit. In our patient, CT imaging was critical in identifying PPT with findings of infectious erosion through bone from the frontal sinus into the orbit (Figure 2C).
In a majority of microbiological studies, Staphylococcus aureus and Streptococcus species are the most common causative organisms of orbital cellulitis.2,11-13 An increasing incidence of methicillin-resistant Staphylococcus aureus has been noted and should be factored into antibiotic management.2,13 Age is also a significant consideration, as patients younger than 9 years with subperiosteal abscesses are more likely to have single aerobes isolated, while older patients trend towards polymicrobial infection with anaerobes.14 Haemophilus influenzae was once one of the most commonly isolated bacteria in children with orbital cellulitis; however, since the advent of the HiB vaccine in 1990, incidence has sharply declined.15,16 H. influenzae is considered relatively aggressive, with a higher association with bacteremia and neurological complications. It’s been hypothesized that its decline may partially explain the decrease in incidence of neurological complications from orbital cellulitis, as less-virulent bacteria now predominate.2,13,17 In immunocompromised or diabetic patients, fungal infections should be considered. Cultures from abscesses and infected sinuses typically provided the highest yield (90 percent) with blood cultures producing low to minimal positive results (0 to 8 percent).10,12
In our patient, Streptococcus anginosis was the predominant organism. S. anginosis falls into a subgroup of S. viridans and is considered normal flora of the human oral cavity and gastrointestinal tract. Despite this, S. anginosus is known for its pathogenicity and propensity for abscess formation with aggressive pyogenic invasion of tissue including the CNS, head, neck and thorax.18 In one genetic analysis of S. anginosus isolates, numerous virulence factors shared with S. pyogenes were identified including genes for antibiotic resistance, superantigens and DNase.19 In one study of 104 patients with complications from sinusitis ranging from orbital cellulitis to cavernous sinus thrombosis and intracerebral abscesses, S. anginosus was the most commonly isolated organism.20 Interestingly, several case series of orbital cellulitis have identified S. anginosus as the most commonly isolated pathogen.21,22 However, in one review of 32 cases with PPT, only two cases isolated S. anginosus and neither case involved the orbit.8 Clinically, S. anginosus infection responds well to cephalosporins, but the potential for resistance exists, and timely surgical drainage is often required.18
Rapid institution of IV antibiotics with appropriate surgical drainage is the mainstay of treatment for orbital cellulitis and Pott’s puffy tumor. Antibiotic therapy may be guided by local sensitivities, but typically a broad-spectrum antibiotic such as a third-generation cephalosporin with consideration for anaerobic and MRSA coverage is an appropriate initial therapy.2 Surgical drainage of orbital and subperiosteal abscesses in orbital cellulitis is often necessary, especially when more severe signs are present, such as decreased vision, pupillary changes, increased IOP or failure to respond to treatment, or in the presence of an orbital foreign body. PPT almost always requires surgical intervention.5 A multidisciplinary approach with functional endoscopic sinus surgery is also typically indicated in cases of PPT or orbital cellulitis with significant concurrent bacterial sinusitis. REVIEW
1. Murphy C, Livingstone I, Foot B, Murgatroyd H, MacEwen CJ. Orbital cellulitis in Scotland: Current incidence, aetiology, management and outcomes. Br J Ophthalmol. 2014;98:11:1575-1578.
2. Tsirouki T, Dastiridou AI, Ibánez Flores N, et al. Orbital cellulitis. Surv Ophthalmol 2017 Dec 15. [epub ahead of print]
3. Kloek CE, Rubin PA. Role of inflammation in orbital cellulitis. Int Ophthalmol Clin 2006;46:2:57-68.
4. Flamm ES. Percivall Pott: An 18th century neurosurgeon. J Neurosurg 1992;76:2:319-326.
5. Ketenci I, Unlü Y, Tucer B, Vural A. The Pott’s puffy tumor: A dangerous sign for intracranial complications. Eur Arch Otorhinolaryngol 2011;268:12:1755-1763.
6. Low SA, Hussain A, Gill HS, Monteiro E, Liu ES. Pott’s puffy tumour presenting as a necrotic eyelid lesion. Can J Ophthalmol 2017;52:1:e25-e28.
7. Nisa L, Landis BN, Giger R. Orbital involvement in Pott’s puffy tumor: a systematic review of published cases. Am J Rhinol Allergy 2012;26:2:e63-70.
8. Akiyama K, Karaki M, Mori N. Evaluation of adult Pott’s puffy tumor: our five cases and 27 literature cases. Laryngoscope 2012;122:11:2382-2388.
9. Tsai BY, Lin KL, Lin TY, et al. Pott’s puffy tumor in children. Childs Nerv Syst 2010;26:1:53-60.
10. Ferguson MP, McNab AA. Current treatment and outcome in orbital cellulitis. Aust N Z J Ophthalmol 1999;27:6:375-379.
11. Chaudhry IA, Shamsi FA, Elzaridi E, et al. Outcome of treated orbital cellulitis in a tertiary eye care center in the Middle East. Ophthalmology 2007;114:2:345-354.
12. Georgakopoulos CD, Eliopoulou MI, Stasinos S, Exarchou A, Pharmakakis N, Varvarigou A. Periorbital and orbital cellulitis: A 10-year review of hospitalized children. Eur J Ophthalmol 2010;20:6:1066-1072.
13.Pandian DG, Babu RK, Chaitra A, Anjali A, Rao VA, Srinivasan R. Nine years’ review on preseptal and orbital cellulitis and emergence of community-acquired methicillin-resistant Staphylococus aureus in a tertiary hospital in India. Indian J Ophthalmol 2011;59:6:431-435.
14. Harris GJ. Subperiosteal abscess of the orbit. Age as a factor in the bacteriology and response to treatment. Ophthalmology 1994;101:3:585-595.
15. Ambati BK, Ambati J, Azar N, Stratton L, Schmidt EV. Periorbital and orbital cellulitis before and after the advent of Haemophilus influenzae type B vaccination. Ophthalmology 2000;107:8:1450-1453.
16. Donahue SP, Schwartz G. Preseptal and orbital cellulitis in childhood. A changing microbiologic spectrum. Ophthalmology 1998;105:10:1902-1905;discussion 1905-1906.
17. Amin N, Syed I, Osborne S. Assessment and management of orbital cellulitis. Br J Hosp Med (Lond) 2016;77:4:216-220.
18. Sunwoo BY, Miller WT. Streptococcus anginosus infections: Crossing tissue planes. Chest 2014;146:4:e121-e125.
19. Babbar A, Kumar VN, Bergmann R, et al. Members of a new subgroup of Streptococcus anginosus harbor virulence related genes previously observed in Streptococcus pyogenes. Int J Med Microbiol 2017;307:3:174-181.
20. Oxford LE, McClay J. Complications of acute sinusitis in children. Otolaryngol Head Neck Surg 2005;133:1:32-37.
21. Quintanilla-Dieck L, Chinnadurai S, Goudy SL, Virgin FW. Characteristics of superior orbital subperiosteal abscesses in children. Laryngoscope 2017;127:3:735-740.
22. Seltz LB, Smith J, Durairaj VD, Enzenauer R, Todd J. Microbiology and antibiotic management of orbital cellulitis. Pediatrics 2011;127:3:e566-572.



