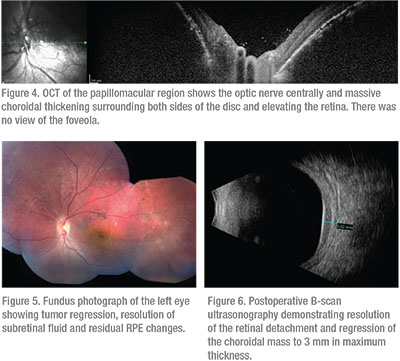
“Tomato catsup fundus” is a term often used to describe the red-orange choroid seen in patients with diffuse choroidal hemangiomas. The patient’s left fundus was suspicious for this (Figure 2) and further diagnostic testing was pursued. B-scan ultrasonography confirmed an inferior retinal detachment and demonstrated a solid echodense choroidal mass of 5.3 mm in maximum thickness, consistent with a diffuse choroidal hemangioma (Figure 3). Optical coherence tomography also demonstrated choroid thickening, and overlying subretinal fluid was noted with no view of the foveola (Figure 4).
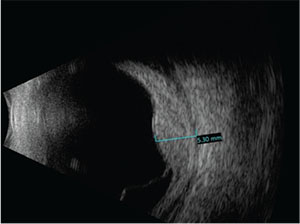 |
| Figure 3. B-scan ultrasound demonstrating an inferior retinal detachment and a solid echodense choroidal mass measuring 5.3 mm in maximum thickness. |
With the addition of the findings on B-scan and OCT, a final diagnosis of diffuse choroidal hemangioma was made. Options for management included external beam radiotherapy, proton beam radiotherapy, plaque radiotherapy and photodynamic therapy. Given his young age and the concern for external radiation exposure, localized plaque radiotherapy was performed.
Treatment
The patient was treated with Iodine-125 plaque radiotherapy. The plaque was positioned directly over the mass posteriorly with transcleral radiation delivery to an apex dose of 35 Gray. To achieve this dose, the plaque was sutured in place for four days. Topical antibiotic/corticosteroid ointment and atropine ophthalmic drops were used for three weeks. At five months follow-up, the tumor had regressed and the subretinal fluid was completely resolved (Figure 5). By ultrasonography, the hemangioma regressed from 5.3 to 3 mm in thickness (Figure 6). The macula was flat on OCT, but there were residual subfoveal RPE changes (Figure 7). Visual acuity remained stable at 20/20 OD and 20/200 OS.
Discussion
Choroidal hemangioma is a benign vascular hamartoma that can be separated into two subtypes: circumscribed and diffuse.1 The etiology of vision loss in choroidal hemangioma can be attributed to foveal distortion, subretinal fluid, intraretinal edema, induced hyperopia, amblyopia or a combination of these factors.2 Diffuse choroidal hemangioma appears clinically as a diffuse, red-orange thickening of the posterior choroid, displaying the appearance of the aforementioned “tomato catsup fundus.”1
The diagnosis of diffuse choroidal hemangioma is often made on clinical fundus examination with indirect ophthalmoscopy, with ancillary testing generally used for confirmation. In cases complicated by secondary total retinal detachment, clinical examination may be limited, so characteristic features of ancillary testing play an important role.3 B-scan ultrasonography demonstrates an echodense dome-shaped mass, often with subretinal fluid.4 On A-scan ultrasonography the mass may demonstrate high internal reflectivity.4 Fluorescein angiography shows diffuse lesion hyperfluorescence in the pre-arterial phase and can be useful in determining the exact site of leakage in large tumors.4 Indocyanine green angiography demonstrates rapid filling of the mass by one minute with washout by 10 to 15 minutes, often with a ring of staining.5
Once the diagnosis of diffuse choroidal hemangioma is made, this should prompt a systemic evaluation for the neuro-oculocutaneous Sturge-Weber Syndrome. Choroidal hemangioma can be present in up to 55 percent of cases.6 The diagnosis of Sturge-Weber Syndrome is established clinically with the presence of a cutaneous capillary malformation (nevus flammeus) in combination with a vascular anomaly of the brain (leptomeningeal hemangiomatosis), best seen on brain MRI.7,8 Associated features include seizures (80 percent),9 focal neurologic deficits (65 percent)9,10 and mental retardation (60 percent).10 The frequency and severity of seizures and neurologic deficits are related to the location and extent of associated capillary venous malformations. Seizure control can be achieved with anti-epileptic medications or surgery if the seizures prove to be refractory to medical management.11
 |
The management of diffuse choroidal hemangioma depends on the extent of the patient’s visual compromise, association with subretinal fluid, and lesion size. Asymptomatic diffuse choroidal hemangiomas can be observed by monitoring for subretinal fluid biannually.4 If the patient is symptomatic, treatment options include photodynamic therapy, external beam radiation therapy, plaque radiotherapy and propranolol.
Laser photocoagulation has been used in the past, with some efficacy for the treatment of circumscribed choroidal hemangiomas. In one study, 62 percent of patients had resolution of subretinal fluid and 71 percent had stable or improved VA.14 However, subretinal fluid limits the therapeutic efficacy of laser therapy and therefore may not be a reasonable treatment option in patients with large diffuse choroidal hemangiomas associated with extensive subretinal fluid. In these patients, treatment with external beam radiotherapy or plaque radiotherapy has been most effective. The primary complications of laser photocoagulation can include RPE atrophy, scotoma and epiretinal membrane formation.14
Photodynamic therapy, like laser photocoagulation, is advantageous as it avoids radiation exposure. Photodynamic therapy also spares the retina and retinal vasculature, compared to other forms of therapy. Also, like laser photocoagulation, it has proven effective in the treatment of circumscribed choroidal hemangioma, with 100 percent of patients achieving resolution of subretinal fluid in one study.15 On the other hand, photodynamic therapy has had limited success in treatment of diffuse choroidal hemangioma, resulting in mixed outcomes, with one case report demonstrating resolution of subretinal fluid and tumor regression after a single treatment16 and another requiring multiple repeat treatments.17
External beam radiation therapy has been used with lens-sparing doses, resulting in good outcomes. Resolution of subretinal fluid occurred in 100 percent of patients in a small study (n=15), tumor regression occurred in five patients, and visual acuity improved in seven. Shortcomings of EBRT include radiation exposure and the need for patient cooperation during treatments.18
Propranolol has also been used with mixed results. While two case studies have shown that oral propranolol can be used effectively in the treatment of exudative retinal detachment secondary to diffuse choroidal hemangioma,19,20 another has demonstrated its failure.21 Propranolol generally fails to induce tumor regression despite the fact that it might resolve subretinal fluid.
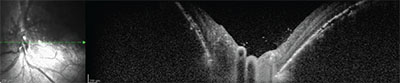 |
| Figure 7. Postoperative OCT of the macula demonstrating resolution of the retinal detachment, a flat fovea and residual RPE changes. |
Plaque radiotherapy is an important treatment option, particularly for patients with extensive subretinal fluid. In a retrospective review of five patients, plaque radiotherapy demonstrated efficacy in both subretinal fluid resolution (100 percent) as well as tumor regression (100 percent) at the 32-month follow-up.22 Visual acuity was stable or improved in four out of the five patients.22 Plaque radiotherapy is advantageous given its application of precise, localized therapy for a short duration (four days), and minimal reliance on patient cooperation.22 We elected plaque radiotherapy for our patient given his young age and our desire to limit the patient’s radiation exposure.22
In the case presented here, the boy had been unsuccessfully treated for amblyopia for five years. His treatment failure, initially attributed to non-compliance, was later found to be the result of a serous retinal detachment secondary to a diffuse choroidal hemangioma. Our patient was successfully treated with plaque radiotherapy and ultimately showed subretinal fluid resolution, hemangioma regression and stable visual acuity.
This case underscores the importance of a thorough examination to evaluate the patient for all possible organic causes of amblyopia prior to assuming that lack of progress or visual regression is due to patient non-compliance with treatment. REVIEW
1. Arepalli S, Shields CL, Kaliki S, et al. Diffuse choroidal hemangioma management with plaque radiotherapy in 5 cases. Ophthalmology 2013;120: 2358-2359.
2. Anand R, Augsburger JJ, Shields JA. Circumscribed choroidal hemangiomas. Arch Ophthalmol-Chic 1989;107:9:1338-1342.
3. Vascular tumors and malformation of the uvea. In: Shields JA, Shields CL, eds. Atlas of Intraocular Tumors. Philadelphia: Lippincott, Williams and Wilkins, 2008:230-251.
4. Turell ME, Singh AD. Vascular tumors of the retina and choroid: Diagnosis and treatment. Middle East Afr J Ophthalmol 2010J;17:3:191–200.
5. Shields CL, Shields JA, De Potter P. Patterns of indocyanine green angiography of choroidal tumors. Br J Ophthalmol 1995;79:237-45.
6. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediat Ophth Strab 1992;29:349-56.
7. Sturge-Weber syndrome: Introduction and overview. In: Bodensteiner JB, Roach ES, eds. Sturge-Weber Syndrome, 2nd edition. Sturge-Weber Foundation, 2010: Mt. Freedom, NJ.
8. Truhan AP, Filipek PA. Magnetic resonance imaging: Its role in the neuroradiologic evaluation of neurofibromatosis, tuberous sclerosis, and Sturge-Weber syndrome. Arch Dermatol 1993;129:2:219-226.
9. Sujansky E, Conradi S. Outcome of Sturge-Weber syndrome in 52 adults. Am J Med Genet 1995;57:35-45.
10. Pascual-Castroviejo I, Diaz-Gonzalez C, Garcia-Melian RM, et al. Sturge-Weber syndrome: Study of 40 patients. Pediatr Neurol 1993;9:4:283-288.
11. Comi AM. Presentation, diagnosis, pathophysiology and treatment of the neurologic features of Sturge-Weber Syndrome. The Neurologist 2011;17:4:179.
12. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediat Ophth Strab 1992;29:349-356.
13. Awad AH, Mullaney PB, Al-Mesfer S, et al. Glaucoma in Sturge-Weber syndrome. Journal of AAPOS 1999;3:1:40-45.
14. Shields CL, Honavar SG, Shields JA, et al. Circumscribed choroidal hemangioma: Clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology 2001;103:12:2237-2248.
15. Blasi MA, Tiberti AC, Scupola A, et al. Photodynamic therapy with verteporfin for symptomatic circumscribed choroidal hemangioma: Five-year outcomes. Ophthalmology 2010;117:8:1630-1637.
16. Anand, R. Photodynamic therapy for diffuse choroidal hemangioma associated with Sturge-Weber syndrome. Am J Ophthalmol 2003;136:4:758-760.
17. Ang M, Shu-Yen L. Multifocal photodynamic therapy for diffuse choroidal hemangioma. Clin Ophthalmol 2012;6:1467-1469.
18. Schilling H, Sauerwein W, Lommatzsch A. Long term results after low dose ocular irradiation for choroidal hemangioma. Br J Ophthalmol 1997;81:267-273
19. Arevalo JF, Arias JD, Serrano MA. Oral propranolol for exudative retinal detachment in diffuse choroidal hemangioma. Arch Ophthalmol 2011;129:1373-1375.
20. Thapa R, Shields C. Oral propranolol therapy for management of exudative retinal detachment from diffuse choroidal hemangioma in Sturge-Weber syndrome. Eur J Ophthalmol 2013;23:6:922-924.
21. Krema H, Yousef Y, Durairaj P, Santiago R. Failure of systemic propranolol therapy for choroidal hemangioma of Sturge-Weber syndrome: A report of 2 cases. JAMA Ophthalmol 2013;131:5:681-683.
22. Arepalli S, Shields CL, Kaliki S, et al. Diffuse choroidal hemangioma management with plaque radiotherapy in 5 cases. Ophthalmology 2013;120:11:2358-2359.



