Outside of the United States, corneal collagen cross-linking has demonstrated long-term ability to stabilize progressive keratoconus and help spare patients the need for corneal transplants.1 The pivotal Phase III study data2 that supported the FDA approval of the Avedro KXL system and the two Photrexas for progressive keratoconus looked at 205 patients at multiple U.S. centers with documented progressive keratoconus, who were randomized into treatment (epi-off cross-linking with Photrexa Viscous and Photrexa riboflavin topical drops and exposure to 3mW/cm2 UVA light) and sham
 |
| Proper alignment of the UV light source is key to success with the KXL system, which requires a corneal thickness of at least 400 µm after removing the epithelium and before irradiance. |
After earning FDA approval for progressive keratoconus in April 2016, the Avedro cross-linking system was approved for corneal ectasia after refractive surgery in July 2016. The pivotal Phase III study3 for that indication randomized 179 patients with documented corneal ectasia post refractive surgery into treatment and sham groups: The treatment group underwent epi-off cross-linking; the sham group got riboflavin without removal of the epithelium or UVA irradiance. In the treatment group, mean KMax decreased by 0.7 ±2.1 D from pre-treatment to one year; mean KMax increased in the sham group by 0.6 ±2.1 D. The CDVA of the treatment group improved by five letters between preop and 12 months, versus a loss of 0.3 letters for the control group during the same interval. As in the pivotal keratoconus study, corneal haze was the most frequently reported adverse finding.
Many Protocols in the Mix
“Cross-linking really changes things, because I would see many more transplants in many more people prior to its introduction,” says Yaron S. Rabinowitz, MD, director of ophthalmology research at Cedars-Sinai Medical Center and clinical professor of ophthalmology at UCLA School of Medicine. “It works so well that a large majority of patients that would have needed a transplant can avoid it because they are not progressing. When the disease is detected in the very young, they progress very rapidly; as such, in those patients, progression is now being retarded with cross-linking,” he explains.
Dr. Rabinowitz is an advocate of the Dresden protocol. He has used a UVA delivery device made by Peschke (Waldshut-Tiengen, Germany) under an IDE from the FDA before and since FDA approval of the Avedro KXL system. Dr. Rabinowitz has been involved in a study comparing cross-linking alone to a combination of cross-linking and INTACs. Patients continue to be enrolled in this ongoing study, which has been conducted for eight years, with another two years remaining to complete enrollment and analysis of data.
Since the FDA approval of the Avedro KXL system and the Photrexa drugs, colleagues at Dr. Rabinowitz’s surgery center now use the Avedro system per the Dresden protocol. Dr. Rabinowitz uses a slight modification of that protocol, which he believes yields better visual outcomes. He uses a VISX laser in PTK mode to remove the epithelium in his cross-linking patients. “When you remove it with a laser, you smooth the center of the cornea, and that actually improves vision as well as removing the epithelium,” he notes. Dr. Rabinowitz plans to present data from his studies at the upcoming 2017 American Academy of Ophthalmology meeting in New Orleans.
Peter S. Hersh, MD, FACS, of the Cornea
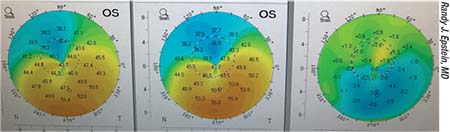 |
| Sagittal difference map showing improved corneal topography of the left eye (far right) after epi-on cross-linking with the CXLUSA photosensitizing agent and UVA light source. |
Brad H. Feldman, MD, an instructor at the Wills Eye Hospital and in practice at Philadelphia Eye Associates, says that since initially offering cross-linking in the spring of 2016, his practice has undergone some major changes. “It has allowed me to finally treat patients in the region who had been losing vision from keratoconus but had no access to this proven treatment to halt disease progression and potentially improve vision,” he says.
“I have had to build in time for lengthy pre-treatment evaluations and discussions about this new treatment,” Dr. Feldman continues. “Many patients have either never heard of the treatment or have misunderstandings about corneal collagen cross-linking. We have also had to change my office schedule to make room for the treatment session, which we perform in the office during a clinic day rather than in the operating room on a separate day. I generally treat on Mondays or Tuesdays, see them postoperative day one, and then remove the bandage contact lens on Friday or Saturday.”
Sometimes, however, things are less than straightforward. “Often, patients have asymmetric disease. This may mean that one eye is going to be monitored for progression rather than treated, or one eye may be too advanced and need keratoplasty,” he explains. Dr. Feldman has also experienced the effect of CXL referrals from other providers, and an increased need for ancillary services related to cross-linking. “Bringing in patients for CXL evaluations––even those who have primary ophthalmologists––has increased the keratoconus population within my practice and increased the volume of related services (keratoplasty, specialty lenses, etc.),” he says.
As an investigator for the CXLUSA Study Group with seven years of cross-linking experience, Randy J. Epstein, MD, CEO of Chicago Cornea Consultants and a professor of ophthalmology at Rush University in Chicago, says the 2016 FDA approval of KXL hasn’t affected his practice. “I’m part of a clinical trial, so I’m not using the FDA-approved version,” he explains. “We’re in an FDA IND study looking at the safety and efficacy of a transepithelial corneal cross-linking system that was developed by Roy S. Rubinfeld, MD.” The current Phase II study (Clinicaltrials.gov identifier: NCT03029104) is enrolling 3,000 patients.
“We’ve treated over 2,000 patients in our center alone,” Dr. Epstein continues. “In that group, we’ve had four retreatments, all of
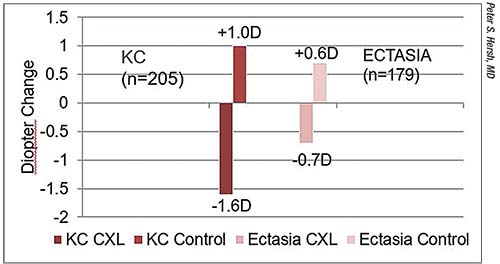 |
| Outcomes of Phase III studies of Avedro’s KXL system in progressive keratoconus (red) and post-refractive surgery ectasia (pink). Treated keratoconic eyes improved by 1.6 D at one year; control eyes continued to progress. In ectatic eyes, Kmax improved by 0.7 D at one year, while control eyes worsened by 0.6 D. |
Dr. Epstein’s group favors transepithelial cross-linking in part because they say it broadens the age range of prospective patients and yields benefits faster than epi-off crosslinking. “By not removing the corneal epithelium, it significantly enhances the benefit-to-risk ratio,” he says.
He credits Dr. Rubinfeld with changing CXLUSA’s protocol to epi-on after a very short period of epi-off cross-linking. “After suffering through a month of epi-off treatments which brought us back to the PRK days of bandage lenses, pain and the whole nine yards, we were very happy to switch to epi-on and never looked back,” Dr. Epstein recalls.
He considers adequate riboflavin absorption more important to cross-linking success than removal or retention of the corneal epithelium. The CXLUSA group’s protocol relies on a customized riboflavin formulation and documentation of adequate riboflavin loading before light exposure to promote this. “The main thing that makes our study different from most previous epi-on studies is that the requirement of our protocol has always been that the surgeon has to assess the cornea after the loading has been completed, to make certain that the cornea has in fact absorbed adequate riboflavin,” he says. “We have a flip chart that has standardized photographs of different degrees of saturation, from grade one to grade five. We aim for a grade of three to four as optimal.”
Treat Sooner? Younger?
Keratoconus is generally a disease that starts progressing sometime in adolescence and stabilizes by the fourth decade of life.4 KXL is currently approved for patients aged 14 years and up with progressive disease, although cross-linking children and adolescents soon after diagnosis without waiting for disease progression may also be safe and beneficial.5
Dr. Rabinowitz has developed criteria that help him decide when to treat.“I use a 1-D change in the steepest part of the cornea; a change in prescription, particularly in the astigmatism; third, patient complaints of worsening vision; fourth, an obvious pattern of keratoconus on topography; and fifth, a change in OCT greater than 20 µm,” he says. “Only patients 14 years of age or older who demonstrate clear progression of disease are candidates for treatment.”
“I have not treated anyone younger than 14 years, but I have treated a 14-year-old. I would consider younger children and create a special consent as needed,” says Dr. Feldman. He weighs the risks and benefits before offering treatment. “I treat when I believe that the patient is at risk for vision loss and that the benefits of CXL outweigh the risks,” he says. “This generally means a patient with a history of recent progression (within the last one to two years). However, in younger patients (<21) I will treat if there is evidence of moderate disease even in the absence of progression, because these patients are at high risk for progression.”
Dr. Epstein advocates treating at younger ages as needed. “Initially, everybody thought that in terms of the actual indication for the procedure, you would have to document progression. But I don’t really think it’s ethical to make that demand for people under the age of 25, or maybe even 30,” he says. “You know it’s going to progress. If it was my kid, I don’t think I would want to sit around and wait for objective evidence of progression before I recommended treatment.
“We can treat people as young as 8 years of age,” he continues. “Most people would not be too excited about treating an 8-year-old with epi-off cross-linking. Ours is a kinder, gentler version of the procedure. We hope that the FDA eventually approves a similar system. In kids, we have the unique ability to stop a disease in its tracks.”
Dr. Hersh concurs that it is better to catch keratoconus earlier when it presents in young patients. “I personally think that it’s important to treat younger people when they are diagnosed, because they are invariably going to worsen over time,” he says. When practicable, Dr. Hersh relies on serial topographies to help him decide when to treat. “One doesn’t simply get KC at 14 and then have it stop,” he says. “It tends to progress more rapidly when you’re younger.”
Managing Expectations
After deciding when to treat, is there a way to predict which patients will respond best to cross-linking? Dr. Hersh and Steven A. Greenstein, MD, published a multifactorial analysis6 of 104 eyes to see if they could find predictors of how individual patients would fare post procedure. “With regard to actual improvement in corneal topography, we found that patients who had steeper cones––patients we defined as 55 D or more––were more than five times more likely to improve by 2 D or more,” he says. “However, we didn’t find any indictor for failure of the procedure to diminish progression. But generally, patients who were worse tended to have a more appreciable improvement. Similarly, looking at corrected vision, most patients stayed the same, but we had about 25 to 30 percent who got more than two lines better; the primary predictor of that was a worse baseline visual acuity. Patients who were 20/40 or worse were about five or six times more likely to improve by two lines or more, compared to those who were better off to start with.
“We also noted a little trend in patients who had very good vision: They were at slightly greater risk of losing one line of vision with the procedure,” adds Dr. Hersh. “I think these are all variables that those beginning cross-linking need to understand and discuss with their patients when determining who should have it done and why they are having it done.”
Although cross-linking can afford patients better visual acuity and function, all four doctors stress to their patients that these are not the definitive goals of treatment. “What we tell patients and their families—and what a lot of my colleagues don’t realize—is that there are three goals of this procedure,” explains Dr. Epstein. “The first goal is to stop the progression. The second goal is to enhance contact lens tolerance. We tell them all going into it that this is our actual objective, because there are a number of studies that have been published showing a fairly
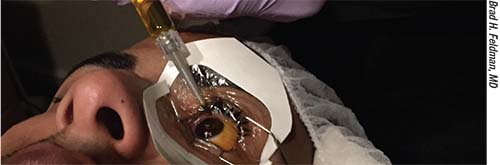 |
| Adequate riboflavin loading prior to UVA light exposure is crucial to ocular safety and a good outcome, regardless of the corneal collagen cross-linking protocol employed. |
Dr. Epstein also notes that most of his patients do report rapid onset of visual improvement, thanks to leaving the epithelium intact. “Most of our patients tell us as early as the next day that they are already seeing better, either with their glasses or when they put on their old contacts, and that’s something we haven’t been able to explain very well,” he says.
“Cross-linking is not designed expressly to help vision, but to halt progression,” stipulates Dr. Rabinowitz. “A small percentage of cross-linking patients do get somewhat improved vision, but I don’t like to tell my patients that. I don’t know who’s going to get improved vision and who isn’t; so if they went in expecting improved vision and they didn’t get it, they would be very disappointed. It’s better for me to tell them, ‘Look, this will stop the progression of the keratoconus, but if you want to make your vision better, then we’ll do INTACs combined with cross-linking.’”
CXL Plus INTACs, Maybe More
As previously mentioned, Dr. Rabinowitz is currently investigating the use of INTACs after cross-linking. “If you combine it with the INTACs, then you actually get improvement of the vision,” he says. “Our study involves comparing a combination of INTACs and cross-linking to cross-linking alone. We’ve found that if you combine the two, the visual outcomes are actually quite a lot better. I have followed some patients for as long as eight years. Follow-up is ongoing––about a 10-year cycle––because we really want to see what happens long-term.”
Dr. Rabinowitz doesn’t perform the INTACs procedure immediately after cross-linking, however. “I like to wait three to six months after doing cross-linking before I do the INTACs,” he says.
Dr. Epstein also adds INTACs after cross-linking to improve patients’ visual outcomes. “If they’re not fittable with contacts, or if they want to take it to the next level visually, they can have INTACs put in after they’re cross-linked,” he says.
“INTACs are meant to change the corneal topography even more dramatically than cross-linking,” says Dr. Hersh. “We do them on a fairly frequent basis because they tackle a problem that KC patients have, separate and distinct from stability, and that is the need to improve corneal topographic asymmetry. Indeed, with the techniques we’re using now, we can achieve upwards of 7 D of improvement in the corneal topography, both flattening the cone and restoring corneal symmetry with improved superior-to-inferior ratio.” Dr. Hersh adds that he’s involved in a clinical trial seeking to determine how well INTACs and cross-linking work together, as well as whether INTACs procedures should be done concurrently with cross-linking, or three months post-CXL.
Cross-linking for infection control, or PACK-CXL (photoactivated chromophore for infectious keratitis cross-linking) is one application that has not yet convinced Dr. Hersh of its efficacy. “With regard to infectious keratitis, published results have been very equivocal thus far,” he says. “It seems that it’s more effective in earlier, more superficial infections, but a lot more work really needs to be done to determine if it’s really efficacious.”
Dr. Epstein’s study colleagues have had some experience with the use of cross-linking for infection control, but he’s skeptical. “We actually had an arm of our earlier IRB study where a number of the centers––ours not included––were doing it for therapeutic indications. The results were not very impressive, and there were just so many confounding factors that I have a hunch that it’s not going to pan out to be a big deal,” he says.
He’s keen on the potential of combining refractive procedures with cross-linking to maximize visual improvement, however. Dr. Epstein relates that one of his patients, after cross-linking and INTACs, went to other surgeons for subsequent toric ICLs and topographically guided PRK to achieve spectacle and contact lens independence. “That shows how far you can carry things,” he says. “We just started doing topographically guided PRK and LASIK treatments with our Wavelight Allegretto laser, and we’re cautiously optimistic that we may be able to use at least some elements of that to at some point start doing custom topographically guided PRK on patients with keratoconus who have previously been cross-linked. That’s really our ultimate goal. That’s off label, and I don’t anticipate an approval. But it’s an exciting possibility,” he says.
Dr. Hersh anticipates the expansion of corneal cross-linking in combination with therapies that will improve vision in newly stabilized eyes. “I think you’re going to find increasing interest in topography-guided PRK and PTK as adjuncts to cross-linking, to further improve the corneal topography. In addition, we’ll do ICLs with great success in many keratoconic patients who are stabilized with cross-linking,” he says. Dr. Hersh is also participating in a study looking at the implantation of preserved corneal tissue to improve corneal topography, thickness and regularity in cross-linked eyes.
Is Access a Challenge?
For all if its promise, many American surgeons are still integrating the FDA-approved cross-linking protocol into their practices—and hoping it will be accessible and economically viable. Effective July 1, 2017, Avedro hiked prices for the KXL system’s two photosensitizing agents, Photrexa and Photrexa Viscous, from $595 to $2,850. Prior to this, a Dutch study attempted to estimate the cost of Dresden protocol cross-linking in the health-care setting, and postulated that the total cost of the procedure for one eye, inclusive of pre- and postop care, was about $1,929.47 ±$194.95.7
“It’s a pretty low-tech procedure: The ultraviolet light is not an excimer laser, and riboflavin is obviously not that complicated of a molecule, so when you get right down to it, it should be something that ought to be widely available at a reasonable price. But there are a lot of factors involved. We do a fair amount of discounting for indigent patients,” says Dr. Epstein, who is involved in a clinical trial and doesn’t use the KXL system or the Photrexa drugs.
“Either insurance companies will choose to set a very high reimbursement fee for the procedure (much higher than what insurance companies have reimbursed thus far), and we will be able to offer the treatment to all patients, or the insurance reimbursements will be too low and providers will only offer this as a cash procedure. This will severely limit access to treatment for the majority of keratoconus patients––as has been the case now for years. This would be a terrible outcome,” opines Dr. Feldman.
“Access is a challenge for patients and for doctors,” adds Dr. Rabinowitz. “Because offering the procedure is expensive, the physician has to do a significant volume to pay for the overhead. It takes a lot of chair time––at least an hour to an hour and a half. I think the whole issue of fees is still evolving.”
Dr. Feldman stresses that guiding preoperative patient expectations and following up postoperatively are costly extensions of the considerable intraoperative chair time that cross-linking requires. “To offer patients the highest quality experience, you need to spend a lot of chair time with them preoperatively and postoperatively,” he explains. “Often, patients arrive believing that CXL is a ‘cure’ that will provide them with normal vision; many believe this is like LASIK. There is almost always worsening of vision transiently after the treatment, with visual improvement typically delayed until several months out. This requires much handholding. Patients who are contact lens dependent are also forced out of contacts for significant periods of time or need re-fits, and this creates a situation where patients are out of work, sometimes for weeks. This again requires plenty of time for communication as well as paperwork.”
Whether and when cross-linking becomes accessible to all patients who could benefit from it remains an open question, but its very availability is a game changer. “Cross-linking is a great new improvement,” Dr. Hersh observes. “It really tackles the most important thing that we need to address in ectatic corneal processes, and that is keeping patients from progressing until they can no longer see well and are in need of corneal transplant. There are also adjunctive procedures that may further improve corneal and visual rehabilitation. There is lot that we’ll be seeing on the keratoconus frontier.” REVIEW
Dr. Rabinowitz reports no financial interests related to cross-linking or any of the associated products mentioned in this article. He has received NIH funding for a study of the genetics of keratoconus through the NEI for 23 years. Dr. Hersh was the medical monitor for the Avedro KXL/Photrexa/Photrexa Viscous clinical trials. Dr. Feldman has not reported relevant financial interests. Dr. Epstein is a consultant for Alcon and Shire, but has no financial interests in products related to cross-linking.
1. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: Ten-year results. J Cataract Refract Surg 2015; 41:41-46.
2. Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK on behalf of the United States Crosslinking Study Group. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology 2017;124;9:1259–1270. Epub. ahead of print. DOI: http://dx.doi.org/10.1016/j.ophtha.2017.03.052. Accessed 7/27/2017.
3. Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK and the United States Crosslinking Study Group. (in press).U.S. multicenter clinical trial of corneal collagen crosslinking for treatment of corneal ectasia after refractive surgery. Ophthalmology 2017. DOI: http://dx.doi.org/10.1016/j.ophtha.2017.05.036. Accessed 8/2/2017.
4. Rabinowitz YS. Keratoconus. Surv Ophthalmol 1998;42;4:297-319.
5. Chatzis N, Hafez F. Progression of keratoconus and efficacy of corneal collagen cross-linking in children and adolescents. J Refract Surg 2012;28;11:753-758.
6. Greenstein SA, Hersh PS. Characteristics influencing outcomes of corneal collagen crosslinking for keratoconus and ectasia: Implications for patient selection. J Cataract Refract Surg 2013;39;8:1133-40.
7. Godefrooij DA, van Geuns P, de Wit GA, Wisse RPL. What are the costs of corneal cross-linking for the treatment of progressive keratoconus? (Letter to the editor.) J Refract Surg 2016;32;5:355.



