The increased prevalence of dry eye, as well as increased awareness of the condition on the part of clinicians and patients, is driving the need to improve our understanding of it. To this end, ophthalmologists and researchers rely on various clinical and lab tests to diagnose dry eye and determine its etiologies. This article will review common dry-eye tests as well as novel diagnostic tools currently used for research purposes.
Objective Measures
• Ocular surface staining. The diagnosis of dry eye relies on dyes that help assess the integrity of the ocular surface. Epithelial damage due to this condition is most commonly measured by applying either fluorescein or rose bengal.1,2 Fluorescein penetrates areas of the corneal epithelium and conjunctival epithelium where intercellular junctions are disrupted (usually first observed nasally), while rose bengal stains damaged areas in which the tear film itself is discontinuous.3
| Figure 1. There is little difference in appearance between rose bengal staining alone (left) and rose bengal combined with lissamine green (right). | |
 |
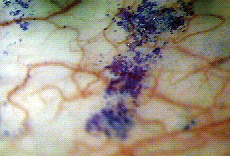 |
Lissamine green has been proposed as an alternative dye for staining. Studies have demonstrated that rose bengal and lissamine green exhibit similar staining patterns in individuals with dry eye (See Figure 1), though lissamine green is less irritating upon instillation.(Krenzer K, et al. IOVS 2000;41: ARVO Abstract 4934)
When evaluating ocular surface staining, the clinician should consider following certain procedures to get the best results. Researchers have shown that the volume of a dye used can influence the determination of surface damage. For example, a large, uncontrolled amount of fluorescein can result in over-saturation of the epithelium, making it difficult to distinguish frank staining from fluorescein quenching.4 Five µL has proven to be a sufficient amount of fluorescein to properly detect damage. You can further highlight staining by using barrier filters such as a Wratten #12 or Tiffen #11 and standardizing the magnification to 16X. The timing of observation is critical. Time to fluorescence can vary from one patient to the next depending on tear volume and turnover rate. Inadequate timing may result in weak or fading surface damage that may be misdiagnosed.4,5 Many agree that the ideal time to measure the presence of staining is approximately five minutes after dye instillation. With lissamine green, 10 µL is recommended, with evaluation one to four minutes later.5
• Tear-film breakup time. One of the more exciting advances in the standardization of objective tools involves a commonly used test known as tear-film breakup time. Traditionally, TBUT has been measured with large and varying amounts of fluorescein (50 µL or more). TBUT has been determined to be greater than 10 seconds in normals and less than 10 seconds in dry-eye patients.1,6
It's been shown that the accuracy and reproducibility of TBUT measurements depend on the amount of fluorescein used. Amounts that exceed the average tear volume (known to be 6-15 mL) probably influence tear-film stability, resulting in an artificially lengthened TBUT. To improve this technique, researchers have used well-controlled, micro-quantities of fluorescein (1 to 5 mL). This has led to more reliable and reproducible reference values. With this technique, TBUT was determined to be greater than five seconds in normals and less than that in dry-eye patients.7
You can instill micro-quantities of fluorescein with either a micropipette or a test strip called the Dry Eye Test (Akorn, Inc.), which delivers 1 mL to the bulbar conjunctiva.8 To further improve the TBUT technique, researchers have developed a digital imaging system with an on-screen timer that provides the most precise measurements.7
• Blink rate. Blinking facilitates the distribution and formation of the precorneal tear film across the cornea.
 |
Several factors influence blink rate: environment (e.g., humidity, temperature, airflow); activities (e.g., personal computer use, reading, conversation); psychological condition (e.g., emotional state, anxiety); and physiological factors (e.g. gender, ocular surface conditions). Any one of these can cause an individual to alter her blink frequency, resulting in an unprotected ocular surface. For example, a recent study showed that blink rate decreased from 17 to 4.5 blinks/min. while patients were reading.9
To properly measure blink rate, it's important that the technique be standardized, consistent and non-invasive. Researchers have developed a precise method for measuring blink rate that incorporates a digital micro-camera and an infrared illuminator that tracks pupil diameter. This device is mounted on a headset and directed towards the eye so that complete blinks (defined as > 95 percent coverage of the pupil) can be measured non-invasively. During the blink-rate evaluation, patients are isolated and asked to complete a visual task.(Casavant J, et al. IOVS 2004;45: ARVO E-Abstract 77) In the absence of this equipment, you can count blink rate while a patient reads the ETDRS chart.
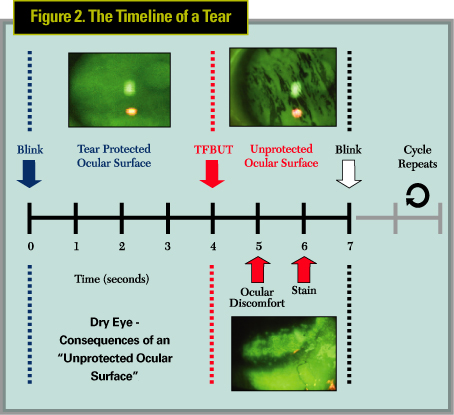
• Ocular Protection Index (OPI). Though TBUT information is reliable and reproducible, there is no consensus on its interpretation and clinical relevance.
When studying the relationship between TBUT and the inter-blink interval (IBI), or the time between blinks, think of their interaction as helping to maintain the integrity of the ocular surface. For example, the ocular surface is protected when the TBUT either matches or exceeds the IBI. In contrast, the surface is unprotected when TBUT is less than the IBI (See Figure 2). This relationship can be clinically relevant since repeated, intermittent exposures of a tear film-deficient cornea lead to ocular discomfort, followed by signs such as keratitis and redness. The discordance between the IBI and TBUT can be worsened by factors that shorten TBUT, such as keratoconjunctivitis sicca and systemic antihistamines, or by factors that lengthen the IBI, such as fatigue and visual tasking.
The OPI has been developed to help quantify the interaction between the IBI and TBUT. The OPI is calculated by dividing TBUT by the IBI. If the OPI is < 1, a patient's cornea is at risk for exposure, and if the OPI is ³ 1, it's not. This approach to measuring alterations in TBUT has proven useful in assessing factors that cause dry eye and evaluating therapies.(Ousler G, et al. IOVS 2002;43: ARVO E-Abstract 56) Agents that modulate the OPI, such as Systane (Alcon), have found wide acceptance among patients.
• Osmolarity. Due to its sensitivity and specificity as a single test or in combination with other tests, tear-film osmolarity may represent the gold standard for diagnosing dry eye, though there are still significant obstacles to its measurement. For example, the technique requires the collection of a large sample of basal tears while avoiding reflex tearing. This is a difficult feat, and an experienced technician is a must. Also, though osmometers are available, there's a demand for an instrument that's more reliable, user-friendly and cost effective.
• Tear secretion tests. The most common test for measuring tear secretion is the Schirmer's test. The standard Schirmer I test is performed without anesthesia and can result in significant variability. Placement of the strip into the cul-de-sac for five minutes can cause irritation and induce reflex tears. The use of anesthesia during the Schirmer's test helps to minimize tearing and improve the test's reliability.4 A less invasive alternative to the Schirmer's test is the phenol red test. The PRT is a piece of cotton thread that is inserted at the lid margin for approximately 15 seconds.
The most effective, reproducible way to measure tear production is fluorophotometry. A fluorophotometer determines tear turnover rate, volume and flow by precisely measuring the decay of fluorescein.10 Researchers have been able to demonstrate the ocular drying effects of a systemic antihistamine by measuring tear production with fluorophotometry (FM-2 Fluorotron Master, OcuMetrics). After only fours days of q.d. dosing, tear volume decreased from 8.61 to 5.12 µL and tear flow decreased from 1.65 to 0.86 µL/min. in normals.11
• Corneal sensitivity. The reduction of corneal sensitivity caused by neurotrophic changes has been documented in patients with herpes simplex keratitis, diabetes and after refractive surgery. Studies have shown that structures such as the lacrimal gland, meibomian glands and goblet cells are highly innervated and that decreased corneal sensitivity can lead to impaired tear secretion and pathological changes in the corneal epithelium. Thus, the ability to monitor corneal sensitivity is critical to understanding the progression of the signs and symptoms of dry eye.
An instrument known as the Luneau Cochet Bonnet Esthesiometer measures corneal sensitivity. It allows clinicians to quantify sensation by changing the length of a nylon filament and the extent of the force applied to the cornea. Dry-eye patients generally have decreased sensitivity.12
• Other measures. Impression cytology refers to the application of cellulose acetate filter material to the eye to remove the superficial layers of the conjunctival epithelium, goblet cells and conjunctival mucus network. This test is used to determine epithelial cell morphology and goblet-cell density, which relate to the amount of mucin on the surface.13 Further, flow cytometry can be performed by extracting cells from the impression cytology samples. These cells are mixed with antibodies specific to the molecule in question, then by an immunofluorescent antibody specific to the initial antibody. A flow cytometer quantifies the number of cells that have bound each immunofluorescent antibody, indicating the number of cells displaying the molecule.14,15
Evidence suggests that the lipid material produced by meibomian glands plays a critical role in the pathogenesis of various ocular surface disorders.16 A new laser meibometer measures meibomian lipid levels on the lid margin. By blotting meibomian gland oil onto a piece of plastic and monitoring its transparency, researchers can calculate the amount of oil and its rate of delivery.17 This may be useful in evaluating meibomian gland function in dry-eye. Examination of the meibomian orifices can provide insight on the lipid layer, such as whether the glands are capped or not.
Tear evaporation can now be monitored with a newly developed evaporimeter that detects real-time changes in evaporation rates and tear-film stability. A ventilated chamber with high-sensitivity microbalance sensors is used to evaluate tear evaporation.18
A method for evaluating tear-film stability using videokeratography, called the Tear Stability Analysis System, is also being used. This system can automatically capture consecutive corneal surface images every second for 10 seconds. These topographies are analyzed for tear-film breakup time and the ratio of breakup area to the entire color-coded area.19
Meniscometry is a non-invasive technique for measuring tear meniscus curvature. The system consists of a camera and a target with a series of black and white strips that are oriented parallel with the axis of the lower tear meniscus. The device is calibrated using a graduated series of glass capillaries of known diameters so the clinician can measure the tear meniscus curvature.20 In the absence of a meniscometer, you can estimate meniscus height at the slit-lamp.
Subjective Measures
• Symptoms. General symptoms of dry eye include dryness, burning, foreign body sensation, stinging, grittiness, light sensitivity and blurriness.
When investigating dry-eye cases, don't provide patients with words to describe their discomfort. This allows them to honestly report their symptoms so you can make a more exact diagnosis. It's also helpful to determine the time of day when symptoms are the worst, the visual tasks that worsen the condition, and which conditions lead to irritation. Stiff hands in the morning or dry mouth can indicate Sjögren's syndrome, for example.
• Quality of life. A valuable instrument used in studies and clinical trials to evaluate dry-eye symptoms is a validated quality-of-life questionnaire. There are a number of available QOL questionnaires, such as the McMonnies and the Ocular Surface Disease Index, that have attempted to characterize dry eye in terms of symptoms, though there may be a need for the development of a new version based on our improved understanding. Researchers recently developed a newer questionnaire known as the Dry Eye Quality of Life Questionnaire, which shows promise as a reliable tool. (Pollard S, et al. IOVS 2004;45: ARVO E-Abstract 82) The sensitivity and specificity of data derived from good questionnaires is a testament to the benefit of standardizing our subjective outcome measures.
• Symptomatic tear-film breakup time. It has been shown that 73 percent of patients experience ocular awareness followed by discomfort within one second of TBUT (Nally L, et al. IOVS 2000;41: ARVO Abstract 1436); this suggests a potentially simpler, non-invasive test of tear-film stability, the SBUT. To do it, have your patient stare straight and monitor the time from his last complete blink to the moment he reports ocular awareness; this time should be within approximately one second of his TBUT. A patient can do this at home, as well.
By combining our understanding of dry eye and the ever-improving technologies from the research realm, our diagnostic tools will continue to improve, allowing ophthalmologists to properly diagnose and treat dry eye.
Dr. Abelson, an associate clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at Schepens Eye Research Institute, consults in ophthalmic pharmaceuticals.
Mr. Ousler is director of the Dry Eye Department at Ophthalmic Research Associates in North Andover.
1. Lemp M. Report of National Eye Institute / industry workshop on clinical trials in dry eye. CLAO J. 1995; 21; 221-32.
2. Bron A, Diagnosis of dry eye. Surv Ophthalmol 2001;45:S221-6.
3. Norn M. Rose bengal vital staining. Acta Ophthalmol (Copenh) 1970; 34:526-59.
4. Foulks G. Challenges in Clinical Trials of Dry Eye Treatments. Ocular Surface. Jan. 2003;1:1:20.
5. Abelson M, Ousler G, Emory T. Dry eye syndrome: diagnosis, clinical trials and pharmaceutical treatment – 'improving clinical trials'. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3 Part A. 2002; 506:1051-1055.
6. Holly F. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515-25.
7. Abelson M, Ousler G, Nally L. Alternative reference values for tear-film breakup time in normal and dry eye populations. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3 Part A. 2002;506:1121.
8. Korb D, Finnemore V, Herman J. A new method for the fluorescein breakup test. IOVS. 1998;40.
9. Tsubota K, Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol. 1995; 113: 155-158.
10. Mathers W. Tear flow and evaporation in patients with and without dry eye. Ophthalmology 1996;103:4:664-9.
11. Welch D, Ousler G, Nally L, Abelson M. Ocular drying associated with oral antihistamines (loratadine) in the normal population—an evaluation of exaggerated dose effect. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3 Part A. 2002;506:1051.
12. Xu P, Yagi Y, Tsubota K. Decrease in corneal sensitivity and change in tear function in dry eye. Cornea 1996; 5:3:235-239.
13. Nelson J. Impression cytology. Cornea 1988;7:1:71-81.
14. Gibson I, Inatomi T. Cellular origins of mucins of the ocular surface tear film. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 2 1998;221-228.
15. Dartt D, Sullivan D. Wetting of the ocular surface. In: Albert DM and Jakobiec FA, eds. Principles and Practices of Ophthalmology, 2nd Ed. Philadelphia: WB Saunders and Co., 2000;960-981.
16. McCulley J, Shine W. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res. 2004;78:3:361-5.
17. Komuro A, Yokoi N, Kinoshita S, Tiffany J, Bron A. Assessment of meibomian gland function by a newly developed laser meibometer. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3 Part A 2002;506:517.
18. Mathers W, Binarao G, Petroll M. Ocular water evaporation and the dry eye. A new measuring device. Cornea 1993;14:4:335-40.
19. Goto T, Zheng X, Tsubota K. A new method for tear film stability analysis using videokeratography. Am J Ophthalmol 2003;135:5:607-12.
20. Yokoi N, Bron A, Tiffany J, Brown N, Hsuan J, Fowler C. Reflective meniscometry: a non-invasive method to measure tear meniscus curvature. Brit J Ophthalmol 1999;83:1:92-7.



