To the Editor:
We were quite unsettled by Charles B. Slonim, MD, FACS, and colleagues' article entitled "Allergy: Understanding the Role of Inflammation" (June 2007, p. 56), which contains both inconsistent and misleading information regarding the late phase allergic response and available topical ophthalmic medications for allergic conjunctivitis. Many of the authors' statements contradict our clinical experience and knowledge of the ocular late phase response (LPR) and available anti-allergy therapies.
Of particular note was the claim that eosinophil infiltration occurs in 25 percent of seasonal allergic conjunctivitis patients and in up to 84 percent of perennial allergic conjunctivitis patients, and a later statement that the LPR occurs in more than 50 percent of individuals with allergic conjunctivitis. Not only do these statements contradict each other, but our clinical experience has shown that the clinical presentation of LPR is a rare occurrence. The authors' reported percentages are misleading, as the references given did not refer to epidemiological or clinical studies. Without the evidence of epidemiological studies on broad populations, it is quite presumptuous to claim that the LPR occurs in half of all ocular allergy sufferers.
The authors also misrepresent the abilities of azelastine and olopatadine on Table 1. The claims of azelastine ophthalmic solution's ability to inhibit platelet-activating factor (PAF) and leukotriene production in the conjunctiva are uncertain, as the referenced studies neither assessed ocular tissues nor examined the full time range of LPR, which, in the eye, usually begins four to eight hours after allergen challenge and can last for 24 hours.1 These studies included alveolar cells,2 peripheral blood samples,3 and rat basophilic leukemia cells,4 with no mention of the investigation of ocular tissue. The presentation of data in Table 1 suggests that azelastine HCl 0.05% ophthalmic solution (Optivar, MedPointe Pharmaceuticals) inhibits PAF and leukotrienes in ocular tissues. In actuality, these findings were shown in alveolar, blood, and basophilic leukemia cells, and as the LPR can vary amongst tissues, the presented results should not be construed as effects that mirror those that occur in the eye. During an LPR that occurs in the lung, airway hyperresponsiveness can last for up to two weeks, and inflammatory cell infiltration, for up to seven days.5 Conversely, the symptoms of the ocular LPR are not very well known; thus, the inclusion of studies evaluating anti-allergy agents on ocular tissues would have been more appropriate than the ones cited by the authors. 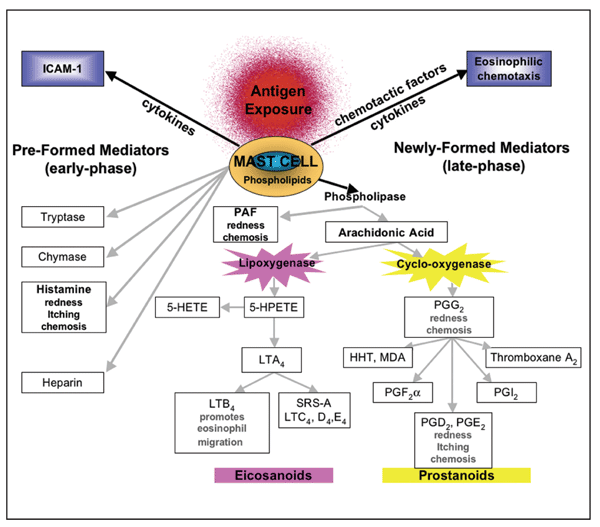
The authors also failed to indicate the full range of effects produced by olopatadine. In previous studies, the molecule has demonstrated PAF and leukotriene inhibition,6 as well as ICAM-1 downregulation7,8 within ocular tissues. Olopatadine significantly suppressed ICAM-1 upregulation, as well as TNF-alpha, which elevates ICAM expression in human conjunctival mast cells that were subjected to anti-IgE antibody for 90 minutes.7,9 A conjunctival allergen challenge clinical study showed that olopatadine decreased the numbers of eosinophils, neutrophils, lymphocytes, ICAM, and histamine in human tears at five hours post-challenge,8 the only study we are aware of that looked at the late-phase response with a drug of this class in allergic patient eyes. Olopatadine has exhibited both in vitro and clinical efficacy in the inhibition of inflammatory mediators.
We acknowledge that some of the information in Slonim and colleagues' article is presented correctly, such as the studies that demonstrate azelastine's abilities to decrease eosinophil infiltration and ICAM-1 expression.10 We also agree with the conclusion that the more pathways of the inflammatory cascade a drug can inhibit, the better; however, any claims of efficacy for controlling late-phase allergic conjunctivitis must be supported by data gathered from experiments in ocular tissues, and when presenting data from different drugs, a full review should be presented in each case so not to skew the reader's interpretation. Furthermore, the prevalence and characterization of the LPR must be accurately represented. Finally, primary references should be cited for claims about a drug's activity; review articles do not substantiate a medication's abilities. The authors referenced many review articles, in vitro and pre-clinical studies, resulting in a selective literature review and omission of well-controlled clinical studies, which are more applicable. Patients and physicians deserve a full picture of the anti-allergy medication market to make an informed decision of what ophthalmic agent might best serve them.
Mark B. Abelson, MD
1. Abelson MB. Acute allergic conjunctivitis. In:
2. Shindo K, Machida M, Hirai Y, Fukumura M. Inhibitory effect of azelastine hydrochloride on synthesis and release of platelet activating factor from human alveolar macrophages. Prostaglandins Leukot Essent Fatty Acids 1997;57(6):561-6.
3. Ventura MT, Giuliano G, Di Corato R, Tursi A. Modulation of eosinophilic chemotaxis with azelastine and budesonide in allergic patients. Immunopharmacol Immunotoxicol 1998;20(3):383-98.
4. Hamasaki Y, Shafigeh M, Yamamoto S, et al. Inhibition of leukotriene synthesis by azelastine. Ann Allergy Asthma Immunol 1996;76(5):469-75.
5. Kariyawasam HH, Aizen M, Barkans J, et al. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med 2007;175(9):896-904.
6. Ikemura T, Manabe H, Sasaki Y, et al. KW-4679, an antiallergic drug, inhibits the production of inflammatory lipids in human polymorphonuclear leukocytes and guinea pig eosinophils. Int Arch Allergy Immunol 1996;110(1):57-63.
7. Cook EB, Stahl JL, Barney NP, Graziano FM. Olopatadine inhibits anti-immunoglobulin E-stimulated conjunctival mast cell upregulation of ICAM-1 expression on conjunctival epithelial cells. Ann Allergy Asthma Immunol 2001;87(5):424-9.
8. Leonardi A,
9. Cook EB, Stahl JL, Barney NP, Graziano FM. Olopatadine inhibits TNFalpha release from human conjunctival mast cells. Ann Allergy Asthma Immunol 2000;84(5):504-8.
10. Ciprandi G, Buscaglia S, Catrullo A, et al. Azelastine eye drops reduce and prevent allergic conjunctival reaction and exert anti-allergic activity. Clin Exp Allergy 1997;27(2):182-91.
11. Ciprandi G, Cosentino C, Milanese M, Tosca MA. Rapid anti-inflammatory action of azelastine eyedrops for ongoing allergic reactions. Ann Allergy Asthma Immunol 2003;90(4):434-8.
Reply:
We appreciate the opportunity to respond to Dr. Abelson's comments. While we do note that Dr. Abelson makes many worthwhile points, it is incumbent for us to point out that we respectfully disagree with his minimization of the importance of the late phase in ocular allergy.
Our clinical experience has taught us that, on the contrary, late-phase allergic reactions in the eye are not a "rare" occurrence. Those of us in clinical practice also recognize that the vast majority of patients who present to our office for therapeutic relief of the signs and symptoms of their ocular allergy are already well into the late-phase of the ocular allergic response. Primary literature from as long ago as nearly 20 years also supports that a late allergic response appears in more than 50 percent of patients undergoing an allergenic conjunctival provocation test.1
It would be highly unlikely to expect ocular allergy to have a completely different pathophysiology than common allergic reactions, such as allergic asthma and allergic rhinitis, in which mediator release in the early phase causes a defined late-phase response. Late-phase ocular responses to allergen challenge have been demonstrated by Ciprandi et al.2,3 Identifying individuals with a late-phase ocular reaction, as defined by the presence of eosinophils in conjunctival scrapings six hours after allergen challenge, was not problematic. These studies also showed inhibition of eosinophil migration with azelastine eye drops in the late phase following allergen challenge.
Table 1 was primarily based on information provided in the Clinical Pharmacology section of the respective product Package Inserts. We agree that it would have been appropriate to footnote Table 1 to identify the source of the displayed comparisons.
We would hope that Dr. Abelson concurs with us that ocular allergy is often under-appreciated and is, therefore, often inadequately recognized and treated. The main goal of our paper was to enlighten the reader to those pathophysiological factors of the disorder that are frequently overlooked by the clinician in the hope that more comprehensive treatment can be offered to the patient.
Charles B. Slonim, MD FACS
1. Ciprandi G, Buscaglia S, Catrullo A, Pesce G, et al. Azelastine eye drops reduce and prevent allergic conjunctival reaction and exert anti-allergic activity. Clin Exp Allergy 1996;27:187-191.
2. Ciprandi G, Cosentino C, Milanese M, Tosca MA. Rapid anti-inflammatory action of azelastine eyedrops for ongoing allergic reactions. Ann Allergy Asthma Imunol 2003;90:434-438.



