Descemet’s membrane endothelial keratoplasty holds the promise of faster, better visual recovery for the right patients. “It’s great,” says Francis W. Price Jr., MD, of Price Vision Group in Indianapolis. “We’re seeing a gradual increase in the percentage of people who are doing DMEK. It’s just been slow to catch on because it’s harder to do.” Here, three experts share their rules for preserving the graft, avoiding complications, and determining when to try something else.
Handle with Reverence
“The biggest consideration in DMEK surgery is minimizing manipulation of the graft so you can have adherence and survival of the graft,” says Mark A. Terry, MD, director of Corneal Services at Legacy Devers Eye Institute in Portland, Oregon. Neda Shamie, MD, cataract, LASIK and corneal surgeon at Maloney-Shamie Vision Institute and an assistant clinical professor at USC Roski Eye Institute in Los Angeles, says, “I jokingly say I have reverence for the corneal endothelial cells; I really hold them in high regard and I do my very, very best to minimize any manual or mechanical damage to the graft.”
Many surgeons use eye-bank-prepared tissue, but unless it’s patient-ready and preloaded, you’ll still need to place it in an appropriate injector. “It’s critical to use the correct delivery system. We used to use plastic injectors which were used for IOL implants,” says Dr. Shamie, who currently uses glass injectors. “But plastic is very damaging to the endothelial cells. Because these grafts scroll in such a way that the endothelial cells are on the outside of the scroll, the plastic rubbing against those cells can be quite damaging.”
The age of donor DMEK tissue also matters, according to Dr. Price. “For surgeons who aren’t doing DMEK frequently, using tissue that’s 60 to 70 years old makes things easier,” he says, adding that most DMEK surgeons use donors aged 50 or older. “Younger tissue curls up tightly, making it harder to get into the right position to put air underneath it and push it up against the back of the patient’s cornea.”
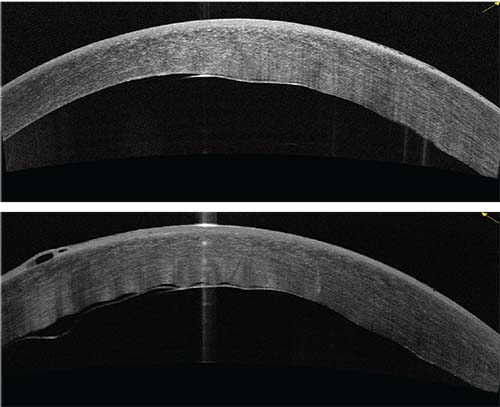 |
| Figure 1: OCT image showing a cornea with central graft separation, six days post DMEK. Vision at the time of this study was 20/400. |
To aid in atraumatic insertion and manipulation, Dr. Price’s DMEK tissue is loaded into the injector in a trifold. “Our tissue is folded Descemet’s out, endothelium in—which is the opposite of how the tissue wants to curl itself. In difficult cases, such as in patients who’ve had vitrectomies, where you can’t shallow the anterior chamber, that can work out a lot better,” he says. “Just pull the tissue in and before letting go, inject air under it so there is no need to shallow the AC.” Dr. Price also says that gentian-violet “S” or “F” stamping is helpful for avoiding upside-down DMEK grafts, although he cautions that the stamping risks some damage to endothelial cells. He prefers to use intraoperative OCT to verify tissue orientation, and it works with thick, hazy corneas.
“I tell visiting surgeons who come to Portland to learn DMEK to choose for their first cases a donor aged 60 or older,” says Dr. Terry. “As you get better at controlling chamber depth and understanding the fluidics of unscrolling the tissue, you can then lower that to 50 years old. As you continue to improve, you can use increasingly younger donor tissue.”
Dr. Terry says that properly orienting the graft starts inside of the injector. “As you inject it, rotate the injector so that the tissue will be right side up at the very beginning,” he explains. “After the tissue’s inside of the eye, then the critical factor is controlling the anterior chamber depth. If your chamber is too deep when you try to unscroll the tissue, you will fail to open the tissue in spite of a lot of manipulation. If the chamber is totally flat, with no fluid in the anterior chamber, and you tap and try to unscroll the tissue, it won’t unscroll then either, and you’ll just damage the endothelial layer. What you want in DMEK surgery is an anterior chamber that you control completely. Anterior chamber depth can’t be serendipitous: The surgeon needs to actively add or remove fluid so that the chamber is very shallow, but not flat.”
“It’s important to keep in mind that the graft is so fragile that if there’s a lot of pressure in the eye during injection, the graft could be ejected from the eye through a tiny 1-mm incision,” Dr. Shamie says. She adds that pupil miosis is critical to preventing trauma inside the eye. “If the pupil is dilated and there’s a plastic IOL sitting right where the graft is injected into the eye, that can be damaging. It’s really important to bring the pupil down as much as possible, since the iris tends to be less traumatic to the endothelial cells.”
Dr. Price points out that in addition to pupil miosis and controlled chamber depth, how gently you perform your fluidic maneuvering of the graft is also important. “You don’t want to do a really hard, long irrigation with balanced salt solution like you would in phaco, because the very thin and pliable DMEK tissue will squirt out of the incision,” he says, adding, “It’s really a lot of fun if done correctly. The donor tissue is like a jellyfish: It goes wherever the flow goes, so you don’t want too strong of a current, just short, gentle bursts of BSS.”
The importance of minimizing touches on the graft cannot be overstated, says Dr. Terry. “Every time the endothelial layer of the donor tissue touches the plastic of the IOL, you’re killing it,” he emphasizes. “This is why you want a small pupil. The key to safe DMEK surgery is to unscroll the tissue without allowing the endothelium to touch any hard object: an IOL, forceps, hook, spatula or anything else.”
If the graft incurs some damage, surgeons say it can be a tough call as to whether to proceed. “A little damage is okay: You just want to avoid major damage. That’s where experience comes into play,” says Dr. Price.
“I’ve never had to abort a case, but I do know of surgeons who’ve decided to abort, or if they have a backup tissue, or a case that’s scheduled later, they move on and then cancel the later procedure,” Dr. Shamie says. “It’s rare, though. What I recommend is to proceed: Go ahead with that graft if at all possible. If the cornea does not clear in one to three months, then consider repeating the surgery.
“If there’s a tear, as long as you have a sufficient amount of graft tissue, you can remove the area that’s been torn and strip it away or just tear it off,” she continues. “Even if you transplant a half-moon-shaped or Pac-Man-shaped graft, as long as the cells on the remaining graft aren’t terribly traumatized, those cells will migrate and cover the area where there are no endothelial cells. You want to decenter your graft in those cases so that the optical zone is covered.”
Block Pupillary Block
Pupillary block after DMEK is potentially serious, but less common than postop graft separation.1 “If it happens, it’s for one of three reasons,” says Dr. Terry. “The first reason is that your inferior peripheral iridectomy was not a complete, full-thickness iridectomy. You cannot just depend upon transillumination through the PI: You must make sure that you’ve excised pigment.”
Even if you’ve done a full iridectomy, a gas bubble can cover it postoperatively, constituting the second cause of pupillary block, according to Dr. Terry. “At the end of surgery we always check that the inferior peripheral iridectomy will be open when the patient is sitting up,” he says. “At the end of every case we put an 85- or 90-percent gas bubble in (a 20-percent concentration of SF6), and then we rotate the eye with forceps downward or we have the patient look down. We can see at that point on the table that the iridectomy will be uncovered in the sitting or upright position. Postoperatively, every hour on the hour until they go to bed, we have patients get up and walk around the room for two or three minutes. It helps them feel better and to avoid DVTs, and it also helps get the gas bubble to clear the peripheral iridectomy to prevent pupillary block.”
Although surgeons can easily remove air if a block occurs right after surgery, Dr. Terry warns that a rarer form of pupillary block is still a risk even days after DMEK. “There is also the possibility of pupillary block occurring three or four days post surgery,” he explains. “If a patient goes into a movie theater, for example, and their pupil dilates in the dark, the gas bubble, which is now smaller, can go behind the pupil. Then when the patient walks out of the theater and into normal light, the pupil constricts, trapping the gas bubble behind the iris. This is a reverse pupillary block, which causes severe pain and pressure. I tell my patients, ‘If you have severe pain, I don’t care if it’s three days or a week after surgery: Call us. Within an hour, we’ll take a look at you and see what’s going on.’ It’s a rare occurrence, but patients need to know about it, and you need to be available to them after surgery.”
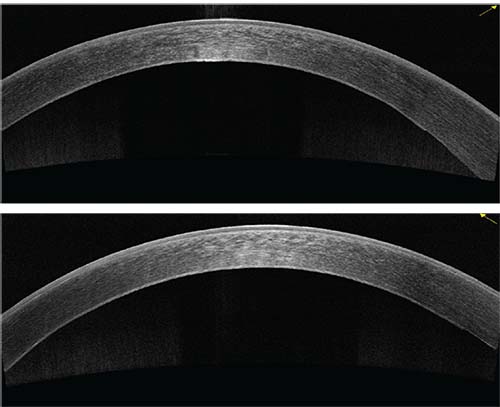 |
| Figure 2: OCT of cornea four days after a rebubble at the slit lamp that was done 10 days post DMEK. The patient achieved 20/20 UCVA. |
Rebubble or Reconsider?
Partial graft detachments are the most common post-DMEK complication;1,2 experienced surgeons can make predictions about a graft’s behavior to aid in decision-making. “I give a graft the benefit of the doubt when I know the surgery went reasonably well and it was as atruamatic as possible,” Dr. Shamie says. “If the graft is not pumping or it’s not attached 100 percent, I tend to feel that it may just need a little extra help. But if it was a graft in which the surgery was truly traumatic, and there was a lot of manipulation of the graft, and I’ve rebubbled once and it’s not clearing or attaching at all, that’s a case where I may not put the patient through waiting three months before giving up on the graft. Rather than letting the patient sit around for three months with blurry vision, I’d probably do a repeat transplant sooner.”
The need for intervention in graft separation depends on degree and location, according to DMEK surgeons. “If there’s any edge lift, if it’s a small amount, you can just watch it,” says Dr. Price. “But if the edema extends into the pupillary area, or shows signs of increasing, then just go ahead and re-inject more air,” he says, adding that his practice’s data indicates that doing two or more rebubblings appears to decrease endothelial cell counts.3 He cautions, however, that DMEK eyes getting repeat rebubblings may have greater cell loss for reasons other than re-injecting air multiple times. “I think that probably makes the data look worse,” he notes.
“Graft detachment is probably the most common risk of DMEK surgery,” says Dr. Shamie. “The key is to decide when to rebubble: If the graft separation is partial, with less than 20-percent separation, you can safely monitor it for spontaneous reattachment of that area.”
If you know your graft was right side up and healthy at the time of surgery, Dr. Terry concurs that watchful waiting is appropriate for partial detachments. “We only reattach a graft with an air-bubble injection if the graft is one-third or more detached or if the central graft isn’t attached,” he says. “That’s our criteria, based on OCT scan.” He cites a paper by Christopher Sales, MD, and colleagues 4 describing a method of reattaching the graft at the slit lamp. “It only takes about three minutes and doesn’t disturb your clinic flow,” he says.
How many rebubbles is too many? “I’ve never had to rebubble more than once,” Dr. Shamie notes. “If you are rebubbling, you should probably try to keep it to less than 20 to 30 percent of the time in cases of DMEK: If you’re rebubbling more frequently, you should probably consider making modifications to your technique in order to lessen trauma to the graft. Similarly, if you find that the majority of your cases require rebubbling more than once, there should probably be reconsideration for modifications. By the time a second rebubble is done, if the graft is not attaching and the cornea is significantly cloudy, then I would consider giving it at least a month before thinking about replacing the graft,” she says. “I wouldn’t give up on a graft, for example, where 50 percent of it is pumping and the overlying stroma is perfectly clear. That’s a graft I would potentially wait on for three months before giving up on it. It’s definitely case-by-case: It’s hard to generalize. The more DMEK experience you gain, the more you know how to anticipate how a given graft will behave, how much time you can consider giving it the benefit of the doubt, and when you should give up on it,” she says.
Reject Rejection
The risk of rejection is low in DMEK, but it does happen.1 “Most often when we see rejection, it’s because people stop taking their drops,” says Dr. Price. “Otherwise, the rejection rate is quite low. Like any other transplant, if you catch it early, in most cases you can reverse it. We’ll typically give more frequent topical prednisone acetate or one of the other steroids. If there’s a significant rejection episode, we’ll give them a one-time dose of IV Solu-Medrol (Pfizer; New York).
Dr. Terry says that it’s important to be alert for other underlying causes of apparent rejection. “It’s very rare that I see a rejection from DMEK surgery. If it occurs, I look at other possible causes of inflammation,” he says. “The key to rejection in DMEK is that it’s usually a silent rejection, so you need to follow these patients closely in the first year. If I see a patient with an increase of white blood cells in the anterior chamber six months after surgery, then I know that the inflammation is not from the surgery itself: It’s either an infectious cause, like a virus such as CMV or HSV, or a true rejection of the tissue that we’ve transplanted. If you see inflammation outside of the early postoperative period—three, four, or six months later—then you should be suspicious not only of rejection, but of possible infectious etiology of the inflammation” he says.
Although DMEK’s role in the corneal-transplant armamentarium is expanding, Dr. Terry urges surgeons not to focus on DMEK to the exclusion of other techniques, since patients may end up needing something else. “The biggest point to be made is that corneal transplant surgeons today should never say, ‘I’m a DMEK surgeon,’ or, ‘I’m a DSAEK surgeon,’ or ‘I’m a PK surgeon.’ If you’re a corneal-transplant surgeon, you’ve got to do it all. We are now in a new era of modern transplant surgery, and you have to be confident using techniques for the entire field.” REVIEW
Dr. Price is a consultant for Haag-Streit, a manufacturer of intraoperative OCT systems. Dr. Shamie is a consultant for CorneaGen of Orange County and Lions VisionGift in Oregon. Dr. Terry reports no financial interests in DMEK surgical devices, but receives royalties from Bausch + Lomb for the design of a surgical instrument used in DSAEK.
1.Deng SX, Lee WB, Hammersmith KM, et al. Descemet membrane endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmol 2018;125:2:295-310.
2.Oellerich S, Baydoun L, Peraza-Nieves J, et al. Multicenter study of 6-month clinical outcomes after Descemet membrane endothelial keratoplasty. Cornea 2016;36:12:1467-76.
3. Feng MT, Price MO, Miller JM, Price Jr FW. Air reinjection and endothelial cell density in Descemet membrane endothelial keratoplasty: Five-year follow-up. J Cataract Refract Surg. 2014;40:7:1116-1121.
4. Sales CS, Straiko MD, Terry MA. Novel technique for rebubbling DMEK grafts at the slit lamp using intravenous extension tubing. Cornea 2016;35:4:582-5.



