Like anti-VEGF injections, cross-linking offers hope to patients who previously had little. And, like anti-VEGF treatments, cross-linking is in a state of rapid evolution. Physicians and researchers around the world are working to improve the procedure so it can produce the best results for the most patients, with the least risk and discomfort.
Here, surgeons with extensive cross-linking experience share the latest ideas and data relating to a host of cross-linking issues.
Leaving the Epithelium On
It’s no secret that having to remove the epithelium (to ensure that sufficient riboflavin reaches the stroma) is the biggest downside of epi-off cross-linking. “Most of the risks connected to the procedure—for example, poor healing, infection and haze—are related to removing the epithelium,” says Kathryn M. Hatch, MD, director of the refractive surgery service at Massachusetts Eye & Ear and an assistant professor of ophthalmology at Harvard Medical School. (Dr. Hatch was an investigator in the CXLUSA study from 2011 to 2016, and says she currently treats 20 to 30 eyes a month using the FDA-approved Avedro system.) “The issue has been getting epi-on to work as well as epi-off.”
To eliminate the need for epithelial removal, several methods for enhancing the passage of the riboflavin through the epithelium have been developed. Some succeeded in getting the riboflavin past the epithelium, but drawbacks became evident, including a regression of the cross-linking benefits between one and two years postoperatively.1,2
Recently, however, several promising new approaches to performing cross-linking without removing the epithelium have emerged. (In addition to the epi-on systems profiled below, Avedro is setting up a clinical trial of its own epi-on cross-linking system. You can find more information about that online at ClinicalTrials.gov, identifier: NCT03442751.)
The CXLUSA system
CXLUSA (mentioned earlier) is a group of American ophthalmologists testing a new approach to epi-on cross-linking. They’re using technology owned by a company called CXL Ophthalmics to perform the new procedure, and some results of their studies have already been published.3,4
Here’s how their approach works: After instillation of proparacaine at the slit lamp, the epithelium is gently brushed with a specially designed sterile sponge soaked with proparacaine to increase epithelial permeability. Then, with the patient in a supine position, another specially designed sponge is placed against the cornea and saturated with a proprietary riboflavin solution. After 10 to 15 minutes the cornea is evaluated to make sure sufficient riboflavin has reached the stroma; if necessary, additional time is spent. Researchers report that most eyes are saturated in 10 to 15 minutes; all were saturated by 30 minutes.
At this point the cornea is rinsed with BSS to remove excess riboflavin. The eye is then exposed to UV light (4 mW/cm2) for 30 minutes, using a proprietary light device that cycles the light on and off, allowing the cornea to re-oxygenate during the dark phase. No additional riboflavin is added during the light exposure.
One of the published studies describes the results of this treatment on 512 keratoconic eyes and 80 post-LASIK ectasia eyes at Woolfson Eye Institute in Atlanta.3 Findings included: No progression (defined as an increase in K-max of more than 1 D and loss of more than one line of CDVA) was observed in any eyes after two years. The epithelium was intact on the first postoperative day, except for rare cases, in which a small, central, epithelial defect was present. Postoperative pain lasted only one day, and visual acuity returned to preoperative levels in two to three days. It produced a significant improvement in UDVA, CDVA, K-max, coma and higher-order aberrations; in fact, the researchers report that the positive effects of this approach equaled or exceeded those of the same measures following the FDA-approved epi-off protocol. In addition, the amount of energy delivered during the procedure was one-third less than that used in the Dresden protocol (3.6 J/cm2 vs. 5.4 J/cm2). Perhaps most important, the benefits didn’t regress by two years postoperatively, as they did with previous epi-on protocols.
R. Doyle Stulting, MD, PhD, director of the Stulting Research Center at Woolfson Eye Institute and professor of ophthalmology, emeritus at Emory University in Atlanta, is a member of the CXLUSA group. (Dr. Stulting performed the first cross-linking procedure in the United States, in January, 2008, as part of an FDA trial that eventually led to approval of epi-off CXL.) “Some physicians are skeptical of this new approach,” he notes. “Since CXL Ophthalmics has not allowed widespread use of its patented technology, others haven’t been able to see the outcomes themselves. In addition, many ophthalmologists are aware that previous epi-on techniques failed to produce lasting results. Mistakenly, they believe that all epi-on crosslinking is the same. This uses a different riboflavin formulation, and the way it’s applied is unique. Also, alternative methods may not have allowed sufficient dark-phase time for diffusion of oxygen into the stroma.
“In any case,” he says, “the clinical trial data indicates that our approach really does work.”
“Custom Fast Cross-linking”
Robert L. Epstein, MD, director of the Center for Corrective Eye Surgery in McHenry, Illinois, has worked with a group in Italy that’s developing a new epi-on protocol pioneered by Ciro Caruso, MD. It differs from the Dresden epi-off protocol in a number of ways, including a riboflavin formulation that incorporates vitamin E to help it penetrate the epithelium. They refer to their method as “custom fast cross-linking,” or cfCXL.
According to one of their published studies,5 this protocol involves no epithelial disruption, 15 minutes of corneal presoaking with a riboflavin-vitamin E solution, and a 370-µm UV beam centered on the most highly curved corneal region. The report notes that the UV beam fluence, total energy and exposure time are all significantly smaller than in the Dresden protocol. This seven-year study, using this method on 81 eyes of 81 patients, found that cylinder was reduced by 26.1 percent at one month postoperatively and 44.2 percent at seven years; best corrected visual acuity improved in 54.3 percent of patients at one month and 82.7 percent at seven years. At year seven, 98.8 percent of patients had no increase in K-max. Average corneal apex flattening was -2.79 ±1.7 D at one month postop and -4 ±2.40 D at seven years.
Dr. Epstein explains that several factors set this treatment apart. “The basic idea is that you want to adjust the energy according to the corneal thickness—i.e., use less energy on a thinner cornea,” he says. “You don’t want to exceed the level that’s toxic to the endothelium.” He notes that cfCXL takes several factors into account that change as corneal thickness changes, including how quickly the riboflavin disappears during treatment. (A paper in Cornea explains the mathematics of this.6)
“Keep in mind that our protocol washes the riboflavin off the corneal surface before UV exposure,” he adds. “That means that less fluence is needed than in the Dresden protocol, because adding riboflavin and leaving it on the cornea in that protocol acts as a barrier, creating a need for a higher UV light energy level. It also adds to the variability of the result. Washing the riboflavin off makes the outcome more accurate and reproducible.”
Comparing the new cfCXL and
CXLUSA epi-on protocols, Dr. Epstein notes several differences. “For one thing, they rub the cornea for 15 seconds with a sponge soaked with their solution,” he says. “They reported that about 5 percent of their patients had small epithelial defects afterwards.3 Our system doesn’t involve any mechanical disruption of the cornea; it’s completely nondestructive. Another difference is that we required documentation of progression before treatment. They did not. In our study, patients were followed for six months before treatment; those that didn’t show progression were excluded. I believe the average preoperative increase in K-max was 2 D among those accepted into the study, so these were very progressive cases.”
 |
Treating With Oral Riboflavin
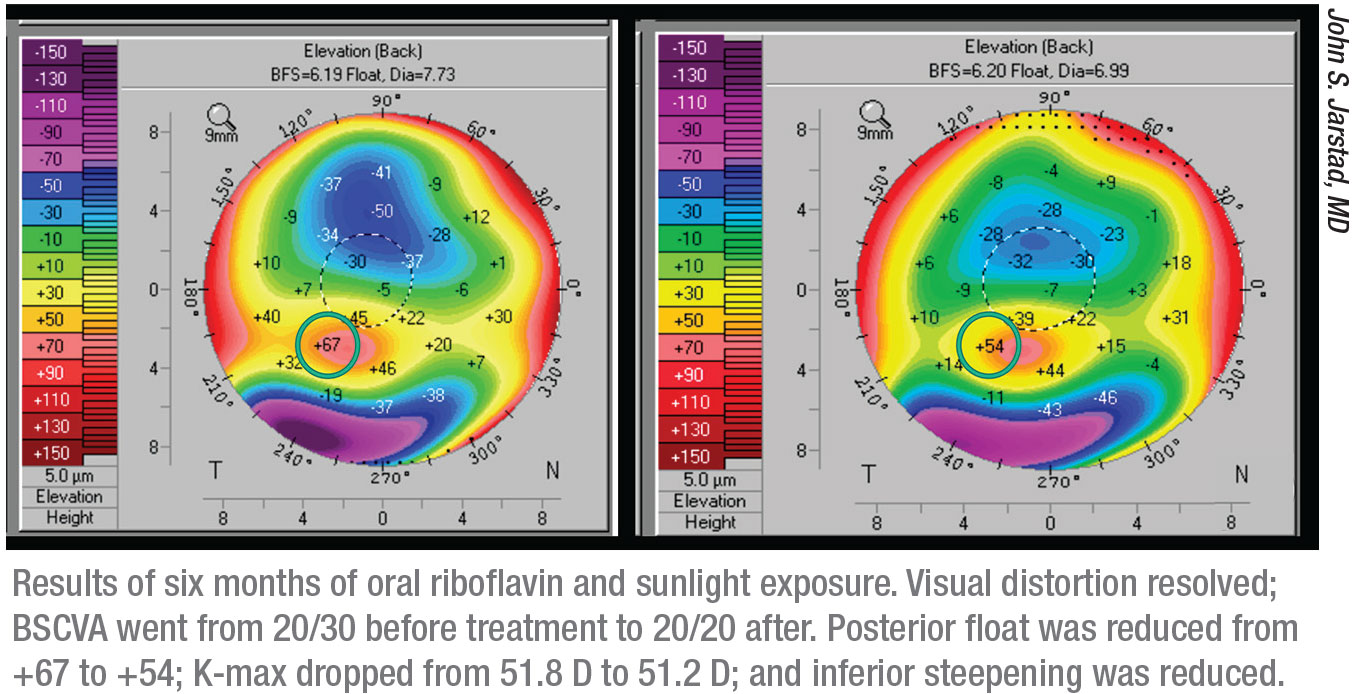
Most variations on the cross-linking procedure center around finding better ways to get the riboflavin into the corneal tissue. Recently, a novel approach was suggested by John S. Jarstad, MD, FAAO, associate professor of clinical ophthalmology and director of cataract and refractive surgery at the University of Missouri School of Medicine. His approach involves the use of an oral riboflavin supplement and taking advantage of the UV light that’s readily available in sunlight. As unlikely as it may sound, the clinical data so far suggests that this approach may be effective.
“About eight years ago we were one of the FDA sites for the Phase I trial of cross-linking,” Dr. Jarstad explains. “A patient came in with post-LASIK ectasia, and we thought she’d be a great candidate for cross-linking. At the time, the cost was $3,500 per eye and it wasn’t covered by insurance. I explained to her why she needed it, and what would be involved—scraping the cornea and so forth. She started crying and asked if we didn’t have something cheaper and less painful.”
He came up with the idea of seeing what would happen if she simply took riboflavin and spent time in the sun. He suggested 50 mg of riboflavin a day and 15 minutes in the sun. “She came back six months later, and to my surprise she had exactly the same results we were achieving with the commercial treatment,” he says. “However, she admitted that instead of taking 50 mg of riboflavin, she’d been taking 500 mg a day for six months.”
After seeing that result, Dr. Jarstad’s group decided to conduct a small study. Those patients all had the same result, so they set up a clinical trial. Dr. Jarstad researched riboflavin and found that there were no reports of toxicity at any level of dosing; even children taking 400 mg/day in a migraine study had no complications. So they chose 400 mg/day as the dose, and added a control group taking 100 mg/day. “Oxygen is key to cross-linking,” he notes, “so we ask our patients to walk briskly, facing the sun without wearing sunglasses between the hours of 10 a.m. and 2 p.m.”
Dr. Jarstad says the results were clear. “The patients taking 400 mg or more—because some patients actually took more—got the best results,” he says. “We presented that data at the international cross-linking conference in 2017 and won the award for the best research.” Since then, they’ve opened the study to sites outside of their geographic area.
In terms of results so far, Dr. Jarstad says the protocol has worked in every single patient that has taken 400 mg or more, except for one patient who wore his hard, UV-blocking contact lenses while walking outside. “The smaller 100-mg dose seems to stabilize the keratoconus,” he says. “Those patients didn’t get worse, but they didn’t get the same amount of improvement. The full-treatment group has had an average of 1.2 D of flattening, which is about what we were getting with the commercial trial for Avedro. Dr. Alan R. Schaeffer, at the University of Tennessee Memphis, reported that a keratoconus patient of his, a clinical dietician who read our research, took 1,500 mg per day. She suffered no ill effects and had flattening of 2.5 D.
“In terms of timing,” Dr. Jarstad adds, “we’ve seen an effect as early as three months, but the best effect was seen after six months, which was the point at which we ended the studies. No adverse effects have been reported. Best of all, the cost is negligible. The riboflavin costs about $4 a month, and the sunlight is free.” He expects to have more data within six months.
Dr. Jarstad says interested parties can find the details about the protocol on the NIH trial website: clinical trial NCT03095235. “We measure visual acuity, keratometry, corneal topography—using the refractive settings on our Pentacam topographer—and pachymetry, before the treatment and at three and six months into the treatment,” he says.
Some surgeons are concerned about potential side effects of the oral riboflavin. “If you get enough concentration of riboflavin in the cornea to cross-link it, what are you doing to the skin?” Dr. Epstein asks. “I think it makes more sense to direct the riboflavin at the organ that’s being treated.”
Dr. Jarstad reiterates that no complications have been seen in his studies, or in association with large doses of riboflavin in the literature. “This protocol could be great for patients who are pregnant, as well as pediatric patients, who tend to regress after the commercial cross-linking,” he says. “I don’t want to discount the existing cross-linking procedure. In some cases that’s the only thing that will work, because you don’t always have time to wait six months for the effect. But this might turn out to be a great adjunct to other methods of cross-linking.”
Using a Stromal Pocket
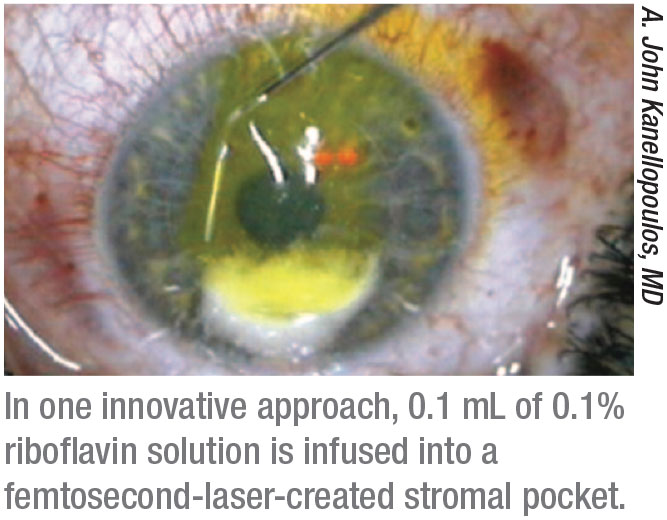 |
A. John Kanellopoulos, MD, a clinical professor of ophthalmology at NYU Medical School and medical director of the Laservision.gr Institute in Athens, Greece, explains that his group developed the idea of introducing the riboflavin into the corneal stroma via a femtosecond-laser-created cornea pocket (similar to that created for the SMILE procedure) as a way to avoid having to remove the epithelium, thus avoiding the associated disadvantages.
“We introduced this concept more than 10 years ago,” he says. “If a cornea doesn’t have advanced disease, the pocket can be created in a relatively simple manner. This approach has very low morbidity and rapid recovery, and, in my opinion, has a significant advantage over other epi-on approaches that administer riboflavin from the surface of the cornea inwards. If you administer the riboflavin through the corneal surface, the riboflavin left in the surface of the cornea will then act like an umbrella, blocking some of the UV light. It will reduce the efficacy of stromal cross-linking and necessitate using more energy to accomplish it.
“We’ve done several ex vivo and in vivo studies showing that this in-pocket epi-on cross-linking technique is very efficient and safe,” Dr. Kanellopoulos adds. “However, it does require the adjunct use of a femtosecond laser, and it can only be performed safely and efficiently in corneas that are at least 400 µm thick that don’t have significant apical scarring.”
Dr. Kanellopoulos also notes a recent innovation. “Investigators have been collecting clinical data regarding placing a very thin allograft of tissue with Bowman’s membrane into the stromal pocket,” he explains. “This idea was pioneered by Gerrit Melles, MD, PhD in the Netherlands. We’ve tried this technique in combination with in-pocket crosslinking and we believe it might be the ultimate cornea-strengthening procedure for corneas thinner than 400 µm—those approaching the point at which a corneal transplant might be their only option.”
Not all surgeons, however, are convinced that creating a stromal pocket is a good approach. “Why slice open a cornea that’s already weak, when you can get the riboflavin in by much less invasive means?” Dr. Stulting asks. “The last thing you want to do is create a way for bacteria to access the corneal stroma, or create a corneal ulcer, corneal scarring or corneal perforation. I believe the risks outweigh any potential benefits.”
 |
Accelerated Cross-linking
Accelerated cross-linking refers to the idea of altering the standard parameters to achieve the same result more quickly—without sacrificing safety. “Accelerated crosslinking is a general term for cross-linking with any fluence greater than the classic 3 mW/cm2 described in the original Dresden protocol,” Dr. Kanellopoulos explains. “The idea is to deliver a similar amount of total cross-linking energy in less time. That reduces patient morbidity, the chance of corneal exposure to airborne microbes and the likelihood of significant corneal dehydration.
“We introduced higher-fluence, or accelerated cross-linking in 2007,” he continues. “We’ve reported on several different techniques, beginning with our introduction of using 6 mW/cm2 and then 10 mW/cm2. Those include ‘LASIK extra’ using 30 mW/cm2, and in-pocket crosslinking using 10 mW/cm2.”
Dr. Stulting points out that the idea of adjusting the parameters to allow a shorter procedure time has some limitations. “These modifications of the original protocol are based on the idea that the effect of a light-based chemical reaction results from the total energy delivered, rather than just the intensity,” he says. “The problem is that this principle originated in reference to some specific non-biological chemical reactions; it’s not meant to apply to biological events. The other problem with this concept is that oxygen is necessary for the most efficient cross-linking reaction. That places a limit on how much you can speed up the procedure, because there’s a limit to the rate at which oxygen will diffuse into the cornea.”
Nevertheless, Dr. Kanellopoulos says they’ve had success with several iterations. “Our ex-vivo work comparing several higher-fluence options has shown that higher fluence is relatively effective up to 30 mW/cm2,” he says. “After that, the procedure isn’t significantly different from a sham procedure.”
In any case, Dr. Hatch believes that customized cross-linking is the future. “Every eye is different,” she points out. “Right now in the U.S. we do the same treatment for every eye that has keratoconus. Customized treatment just makes sense.”
400 µm of Tissue?
The accepted wisdom that cross-linking should only be done on corneas at least 400 µm thick has recently come into question.
Dr. Kanellopoulos notes that the 400-µm cutoff has been around for a while, and its safety has been established. “However, we’ve reported treatments down to almost 350 µm,” he says. “Many other investigators have also established that cross-linking can be done safely at 350 µm. In fact, we’re already using 350 µm as our cutoff—with the proper informed consent of the patient and family, of course.”
Dr. Stulting also believes the 400 µm safety cutoff is no longer supported by the evidence. “This idea was first proposed by work in the laboratory of Theo Seiler, MD, back in 2003, and is based on experiments with rabbit corneas,” he notes.8 “More recently, Åsa Morén, MD, in Sweden, tested endothelial toxicity by directly exposing the endothelium to UV light from the endothelial side. Then he cultured the endothelial cells and looked for toxicity. He found that the threshold for cell death was orders of magnitude higher than that obtained by the original safety calculations.9 This is important, because the whole world is avoiding treating corneas less than 400 µm thick in patients that desperately need treatment, based on data that are probably not correct.”
Dr. Kanellopoulos adds that one way to lower any potential risk when treating a thinner cornea is to simply lower the amount of energy delivered. “For instance,” he says, “if we’re going to cross-link a cornea that’s only 350 µm thick, we deliver 4.5 J of energy instead of our standard 6 J, to avoid cross-linking very deep in the cornea and reaching the endothelial level.”
Iontophoresis
“Iontophoresis—using very mild electric current to draw the large riboflavin molecule from the surface into the cornea stroma through the intact epithelium—is an old idea,” says Dr. Kanellopoulos. “It was pioneered in Italy, and it appears to have reasonable results, although it’s not as popular as it was several years ago.”
Dr. Epstein notes that published results using iontophoresis have been mixed. “A two-year study by ophthalmologist Marco Lombardo in Italy involving 34 eyes of 25 patients compared cross-linking using trans-epithelial cross-linking to standard cross-linking,” he says.10 “Clinically significant improvements were found in both groups, but standard cross-linking resulted in more significant corneal apex flattening. Another two-year study by Guzel Bikbova at the Ufa Eye Research Institute in Russia involving 149 eyes of 119 patients found that iontophoresis was less effective than standard cross-linking at two years, although the paper notes that both methods stopped the progression of the disease.”11
Dr. Stulting points out that using iontophoresis for cross-linking has several drawbacks. “It’s uncomfortable; it’s destructive to the epithelium; and it requires extra equipment to perform,” he says. “I don’t know anyone who’s tried it and decided to continue to do it.”
Contact-lens-assisted CXL
“Contact-lens-assisted cross-linking is a brilliant idea that comes to us from Soosan Jacob, MS, FRCS, of India,” Dr. Kanellopoulos explains. “The idea is to protect a cornea thinner than 400 µm from excessive UV radiance during cross-linking by adding a 50-µm thick, non-UV-absorbing contact lens to the front of the cornea. When the lens and cornea are soaked with riboflavin, the contact lens acts as a buffer, preventing cross-linking in the endothelium. I think it’s a very good idea that has clinical application for corneas under the 400 µm minimal thickness.”
Dr. Stulting notes that the premise—using a contact lens to increase the total thickness to 400 µm or more for safety reasons—depends on accepting the 400-µm limit as accurate. “If you don’t buy the 400 µm safety limit to begin with, then you don’t have to worry about using the contact lens in those eyes,” he says.
Intracorneal Ring Segments
Many corneal surgeons have implanted ICRs—usually Intacs (Addition Technology, Freemont, California)—in pockets created in the peripheral cornea of ectatic eyes in order to flatten the central cornea, thus reducing myopia and astigmatism. Combining this procedure with cross-linking has been seen as a way to preserve the resulting changes. However, some surgeons have reported mixed experiences using Intacs in the cornea in combination with cross-linking.
Recently, some surgeons have begun trying a new variation on this idea, using intracorneal segments made of biological tissue rather than plastic (an idea pioneered by Soosan Jacob in India). Dr. Epstein is one of the surgeons exploring this option. “We create the rings using eye-bank corneas,” he explains. “We use a special blade to cut them at whatever size and length we need, and we can make multiple segments from a single cornea.
“Using biological tissue has a number of advantages over inserting plastic segments into the cornea,” he continues. “For example, if plastic segments are in the cornea and patients rub their eyes, the plastic doesn’t bend, but the cornea does. If enough eye rubbing occurs, the Intac can start to dislocate. That’s not the case with an insert made of tissue. A second advantage is that a biologic insert can be placed inside a thinner cornea than an Intac can. And third, a biologic insert becomes part of the body when it heals. If you laser the corneal surface later, you’re not going to run into plastic.”
Dr. Kanellopoulos says he believes this idea is promising. “Our group has also reported using xenograft tissue in conjunction with cross-linking,” he says. “We believe this may bypass some of the practical issues that come with using artificial intracorneal ring segments. Those issues include obstructing the nutrients and other materials that are transferred in the cornea, and the possibility of the rings making the cornea unstable, creating corneal melts, inviting infection and leading to other complications. Using xenograft tissue instead accomplishes the same thing with a material that’s more biocompatible.”
Treating a Smaller Area
One idea that’s being tried with some success is cross-linking less corneal tissue outside the optical zone. This is showing promising results in both epi-off and epi-on protocols.
Dr. Epstein says this is part of the custom fast cross-linking procedure (described earlier). “We use a narrow-er beam of light,” he explains. “In most cases of keratoconus, the localized area gets very highly curved, while areas distant from it actually become flatter. What we want to do is treat the area that’s diseased, not the area that’s already extra flat. By focusing the beam, we get a much greater effect, even though we’re using less energy than in the past. It’s because the treatment area is smaller, which strengthens the effect on the curvature.”
Dr. Hatch also reports success using this approach with the epi-on protocol, in part because it requires debriding a smaller area. “I typically don’t do a full 9-mm debridement of the epithelium,” she explains. “Instead, I do a more limited debridement over the area of the cone. Sometimes I find that I can get a deeper cross-linking effect in that limited area, and it leads to a much faster recovery.
“You probably won’t get as much flattening as if you did the Dresden protocol,” she adds, “but I’m not sure every patient needs that level of cross-linking. Meanwhile, the epithelium heals in about two days, which is more like the recovery from an epi-on procedure, and you still get a very effective cross-linking treatment.” REVIEW
Dr. Kanellopoulos is a consultant for Avedro and Alcon. Dr. Hatch is a consultant to Avedro and a shareholder. Drs. Epstein, Jarstad and Stulting report no relevant financial ties.
1. Caporossi A, Mazzotta C, Paradiso AL, Baiocchi S, Marigliani D, Caporossi T. Transepithelial corneal collagen crosslinking for progressive keratoconus: 24-month clinical results. J Cataract Refract Surg 2013;39:8:1157-1163.
2. Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: A randomized controlled trial. Am J Ophthalmol 2015;159:5:821-828 e823.
3. Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg 2018;44:11:1363-1370.
4. Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal. J Cataract Refract Surg 2018;44:2:237-242.
5. Caruso C, Epstein RL, Troiano P, et al. Topography and pachymetry guided, rapid epi-on corneal cross-linking for keratoconus: 7-year study results. Cornea 2019 Jul 24. Epub ahead of print.
6. Caruso C, Epstein RL, Ostacolo C, Pacente L, Troisi S, Barbaro G. Customized corneal cross-linking—a mathematical model. Cornea 2017;36:5:600-604.
7. Epstein RL, Chiu YL, Epstein GL. Pentacam HR criteria for curvature change in keratoconus and postoperative LASIK ectasia. J Refract Surg 2012;28:12:890-4.
8. Wollensak G, Spoerl E, Wilsch M, Seiler T. Endothelial cell damage after riboflavin-ultraviolet-A treatment in the rabbit. J Cataract Refract Surg 2003;29:9:1786-90.
9. Mooren P, Gobin L, Bostan N, et al. Evaluation of UVA cytotoxicity for human endothelium in an ex vivo corneal cross-linking experimental setting. J Refract Surg 2016;32:1:41-46.
10. Lombardo M, Serrao S, Lombardo G, Schiano-Lomoriello D. Two-year outcomes of a randomized controlled trial of transepithelial corneal crosslinking with iontophoresis for keratoconus. J Cataract Refract Surg 2019;45:7:992-1000.
11. Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol 2016;94:7:e600-e606.



