As everyone knows, the “holy grail” in cataract surgery is an implant that can give patients back a full range of visual accommodation—the kind most of us enjoyed in our youth. A number of implantable lenses currently in development (not yet approved in the United States or elsewhere) are showing the promise of at least coming close to that goal.
Here’s the latest on the three frontrunners that may give surgeons a true accommodating lens in the years ahead.
The Juvene
The Juvene accommodative IOL (LensGen, Irvine California) was recently profiled at the annual meeting of the American society of Cataract and Refractive Surgery by Eric Donnenfeld, MD, a clinical professor of ophthalmology at New York University Medical Center and a partner at Ophthalmic Consultants of Long Island. (Dr. Donnenfeld is a consultant for LensGen.) He implanted his first Juvene in March of 2018, in Santa Domingo in the Dominican Republic.
“The Juvene is a modular, curvature-changing, fluid-optic IOL that’s been in clinical trials in the Dominican Republic and Mexico for four years,” he explains. “It’s a two-part lens composed of a base component that fills the capsular bag and a modular second implant that contains a curvature-changing liquid silicone optic. It’s biomimetic, meaning that it mimics the natural crystalline lens; when the ciliary muscles contract, the zonules relax and the lens becomes rounder, just like the natural lens. Also, like the natural lens, the Juvene doesn’t split the incoming light the way a multifocal lens does. For that reason, vision quality is excellent and optical side effects are minimal.”
 |  |
| The Juvene accomodative IOL is a two-part lens composed of a base component that fills the capsular bag and a modular second implant that contains a curvature-changing liquid silicone optic. | |
Dr. Donnenfeld notes that filling the capsular bag is an advantage. “Because it fills the capsular bag there’s no change in the effective lens position and no rotation when it’s implanted,” he says. “There’s no posterior capsular opacification, and there are fewer flashes and floaters because the vitreous face doesn’t come forward. Meanwhile, because of the modular components, it can be inserted through a small 3-mm incision. The two-part design allows the platform to potentially be used to address issues such as astigmatism via a toric lens, or to deliver drugs inside the eye or perform electronic sensing. Furthermore, we can take the lens section out very easily, so we can come back five years after implantation and exchange it if something better comes along.”
Sumit Garg, MD, vice chair of clinical ophthalmology, medical director and an associate professor of cataract, corneal and refractive surgery at the Gavin Herbert Eye Institute at the University of California, Irvine, agrees that Juvene being a modular lens is advantageous. (Dr. Garg is also a consultant to LensGen.) “The two-part lens consists of a fixed ‘base’ lens and a fluid-filled ‘power’ lens,” he notes. “In early clinical trials, the lens delivered up to 3 D of continuous range of vision, with minimal or no visual side effects. Because the bag is completely filled, there have been no reports of posterior capsular opacity out to four years. Also, with the bag filled, there should be less stress on the vitreous. That has the potential to confer safety advantages with respect to posterior vitreous detachment and retinal tears.”
In terms of contraindications, Dr. Donnenfeld says that the Juvene is similar to a standard monofocal lens. “Anyone who can accept a monofocal lens can have this lens implanted,” he explains. “You have to have an intact capsular bag, the capsulotomy has to be good and there has to be good zonular support, but these are the usual concerns with any patient having cataract surgery. Indications for this lens would be the same as for a multifocal lens, except that you can put this lens in patients who have keratoconus, for example, or glaucoma. There are no major contraindications, because it functions as a monofocal lens. There’s no splitting of light, so you don’t have to worry about any loss of contrast sensitivity.”
Dr. Garg adds that he’s had the opportunity to implant a few of the
Juvene IOLs. “I found the procedure to be very intuitive, without a significant learning curve,” he says. “The modular lens was easy to implant and manipulate within the eye, and the incision didn’t require any sutures.”
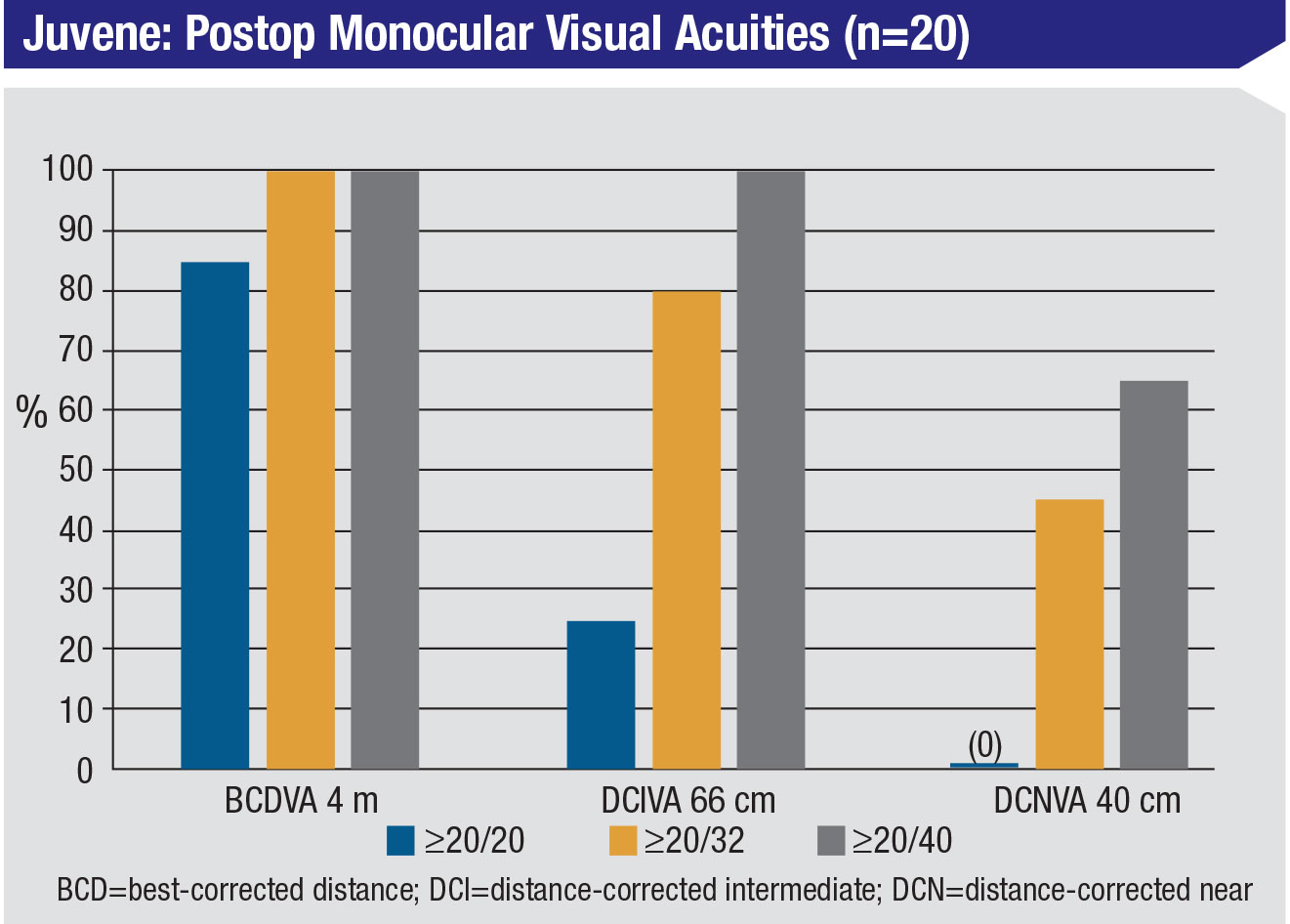
Dr. Donnenfeld says that the lens design has been through a half-dozen different iterations. “Once they finalized the lens design they started a clinical trial in Mexico called the ‘Grail’ study, involving 44 eyes and multiple surgeons,” he says. “At the recent annual meeting of the American Society of Cataract and Refractive Surgery I presented the one-month follow-up data from that study. The data shows that the lens creates a very reproducible 2.5 D of accommodation, and the subjects report excellent quality of vision. With binocular lens implantation, near vision was even better; subjects achieved 3 D of accommodation.
“The great majority of patients were 20/20 at distance,” he continues. “They had excellent intermediate vision, and about half of the patients ended up seeing 20/32 at near. The results were very close to emmetropia. The effective lens position was very good. Patient satisfaction was excellent, with no patients reporting glare or halos, although two patients reported very mild starbursting, the kind you might see with a standard monofocal lens.”
 |
Dr. Donnenfeld also says that four-year data is now available for the first patients implanted with the lens. “Those patients are doing very well,” he says. “None of them developed posterior capsular opacification, because the leaflets of the capsule are separated.”
Asked about similarities to the FluidVision accommodating lens (see page 16), Dr. Donnenfeld notes that they share the same type of mechanism. “They’re both biomimetic, and they both have silicone optics that change as the patient accommodates,” he says. “The major difference is that the Juvene is a modular lens, so it can go in through a smaller incision.”
Dr. Donnenfeld acknowledges that there’s still plenty of work to be done before the Juvene reaches the marketplace. “The FDA trials will start later this year,” he notes. “Meanwhile, the Grail study will involve a one-year follow-up. If we see that the data is just as good with this version of the lens at one year as it was at one month, then we’ll feel much better about it. In the meantime, I remain cautiously optimistic. I think it’s a very exciting lens.”
The Lumina
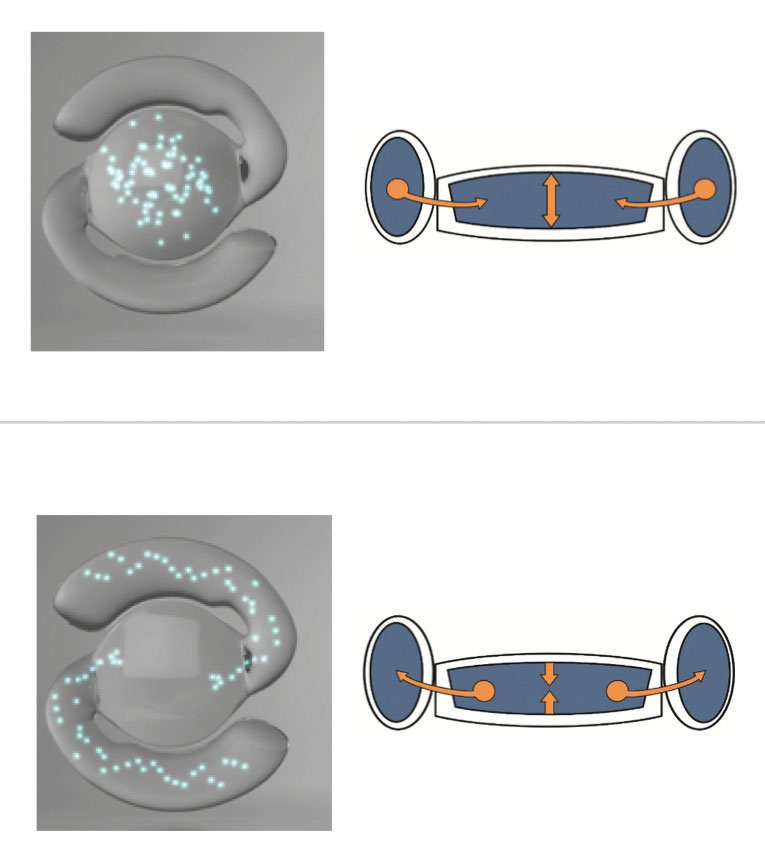
The Lumina accommodative IOL (Akkolens International, the Netherlands) consists of a fixed-power lens that corrects for the refractive error of the aphakic eye, the way a monofocal lens would, and a variable-power lens. The latter is composed of two cubic, progressively powered hydrophilic acrylate optical elements, elastically connected at the rims, that move across each other under pressure from the ciliary muscles. That movement causes a change in focal power. The Lumina is implanted in the sulcus—not in the bag—through a 2.8-mm incision using a standard butterfly-type lens injector. (You can see a Lumina being implanted at youtube.com/watch?v=2nvaQJaF_BI.) The device has rounded anterior edges to prevent release of iris pigment.
 |
 |
| The Lumina accommodative lens consists of a fixed-power lens and a variable-power lens. Two optical elements in the latter part of the device move across each other under pressure from ciliary muscle changes, shifting the focal power from distance to near and back. The Lumina is implanted in the sulcus, not inside the capsular bag. |
Once in the sulcus, the haptics directly contact the ciliary muscles. When the muscles contract because of an accommodative stimulus, the Lumina is compressed by the centripetal force of the muscle contraction. The Lumina’s two optical surfaces then slide over each other, altering the refractive power of the dual lens to provide sharp vision at near. Relaxation of the ciliary muscle reverses the process due to the inherent, outward, elasticity of the IOL, for sharp vision at far. The Lumina can provide a focal range of 3 to 4 D when shifted. (The company reports that the shift in position of the two pieces can be seen in ultrasound images.)
Clinical investigators have noted that placing the lens in the sulcus eliminates several factors that might affect the performance of other accommodating lenses that are placed inside the bag, such as less-direct transfer of the ciliary muscle movement, and gradual capsule shrinkage and fibrosis that may restrict a lens’s accommodative amplitude. (After the Lumina is implanted, standard YAG can be applied to treat posterior capsular opacity should it occur, with full recovery of visual acuity.)
Several studies showing both subjective and objective accommodation have been conducted to date.1,2 Early data from 59 eyes of 43 patients implanted with the Lumina, reported by Jorge Alió, MD, PhD, professor and chairman of ophthalmology at the University Miguel Hernandez de Elche in Spain, and medical director of the investigational study of the Lumina, found that it retained its full range of objective and subjective accommodation for at least two years. At both 12 and 24 months, the subjects displayed an accommodative range of about 3.1 D, although there was some variation among individual patients. According to Dr. Alió, pseudo-accommodation was responsible for less than 30 percent of this result. The company says that long-term stability has been shown by three- to four-year postop evaluations, with patients showing accommodation and excellent contrast sensitivity curves, comparable to monofocal curves, and patients being spectacle-free.
Dr. Alió is the principal investigator for the lens; he performed all of the surgeries and follow-up measurements. He emphasizes that placing the lens in the sulcus is a significant advantage. “Anything that’s placed inside the capsular bag is condemned to become fibrotic and blocked over time,” he says. “Fibrosis is unavoidable, as we demonstrated in our 2015 paper published in the Journal of Refractive Surgery.3 You have a maximum of six months [of full motion] during the follow-up, due to capsular bag shrinkage and contraction. On the other hand, our studies have shown that the Lumina works even if the capsular bag is broken during surgery (which happened in one case), and following YAG laser capsulotomy.”
Asked about potential limitations of this technology, Dr. Alió explains that the range of accommodation appears to depend on the anatomy of the eye. “The evidence that we have demonstrates that it accommodates from 1.5 D to more than 4 D,” he says. “The main limitation is the variability from eye to eye. That’s the only pitfall.” Dr. Alió adds that his accommodative lens group is the only one reporting clinical outcomes in peer-reviewed journals. “I challenge the others to publish what they have shown in meetings,” he says. “My bet is that intracapsular lenses will not work.”
The company says it’s currently developing new iterations of the Lumina that will allow it to pass through an incision of 2.2 to 2.4 mm. Also in the works are a yellow-tinted Lumina lens for blue-light filtering; a toric Lumina lens; an “add-on” Lumina unit that can be added to existing monofocal lenses to restore accommodation; and a pre-loaded injector.
In the meantime, the Lumina is now beginning large clinical trials in Spain and South America. The company says that approval in Europe is expected in the fourth quarter of 2019, followed by expected commercial expansion in Europe and other countries that accept the European approval. FDA testing is expected to begin in 2020. Dr. Alió says that he hopes the Lumina lens will be available commercially in about two years.
The FluidVision Lens
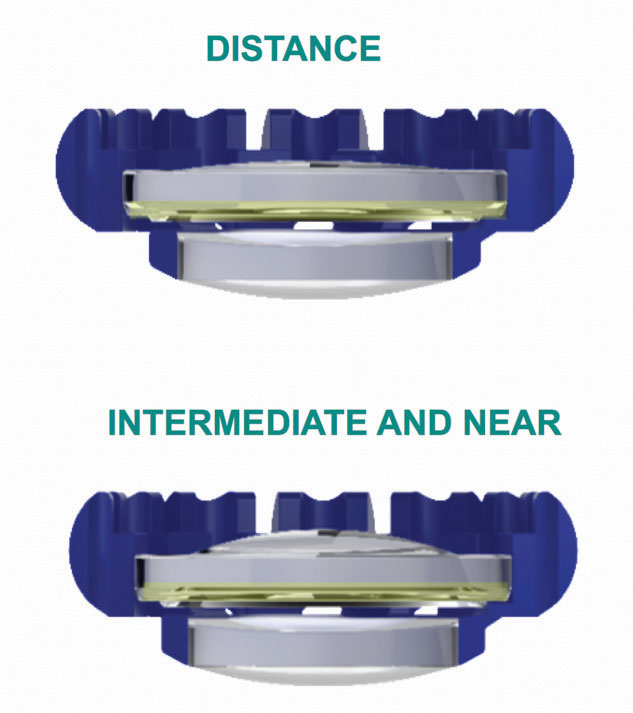
The FluidVision Lens (PowerVision/Alcon) is an acrylic device. The entire lens, including the haptics, is hollow and filled with liquid silicone, which allows the lens to change shape in response to ciliary muscle contrac-tion and relaxation. As the ciliary muscles tighten, a small amount of fluid in the haptics is pushed into the optic section of the lens, causing the anterior curvature of the optic to increase, enhancing near vision. When the muscles relax, the reverse occurs. Earlier versions of the lens required a 3.5-mm incision for implantation; the latest version of the lens with a new injector is able to be implanted through a 3.2-mm wound.
 |  |
| The FluidVision accommodative lens is hollow and filled with liquid silicone—including the haptics. As the ciliary muscles tighten, a small amount of fluid in the haptics is pushed into the optic section of the lens, causing the anterior curvature of the optic to increase, enhancing near vision. When the muscles relax, the reverse occurs. | |
The current version of the device, called the NextGen 20/20 model, is currently undergoing a multicenter international clinical trial. Six-month data, reported in the fall of 2018, found good visual acuity at every distance, with an average accommodation range of 2 D. Louis D. Nichamin, MD, at the European Society of Cataract and Refractive Surgeons meeting in September 2018, reported data from 28 eyes that underwent monocular implantation with the lens at six sites in South Africa. Distance vision was 20/20; intermediate was close to that; and near visual acuity ranged from 20/22 to 20/27. He also reported that an in-house autorefractor detected an average of 2 D of accommodation, with some eyes achieving as much as 5 D of accommodation.
Other randomized, controlled, multicenter studies are currently underway. The ORION study is comparing binocular implantation of the FluidVision lens to monofocals in 54 patients at seven sites in South Africa. The CLEAR study is comparing the FluidVision to trifocal intraocular lenses at multiple centers in multiple countries. Meanwhile, the company is developing a prototype toric version of the IOL, and is working on a model that would be refraction-adjustable after implantation. The latter may be implantable through a 2.8-mm incision. REVIEW
1. Alio JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina accommodative intraocular lens. Am J Ophthalmol 2016;164:37-48.
2. Alió JL, Simonov AN, Romero D, et al. Analysis of accommodative performance of a new accommodative intraocular lens. J Refract Surg 2018;34:2:78-83.
3. Alió JL, Ben-Nun J. Study of the force dynamics at the capsular interface related to ciliary body stimulation in a primate model. J Refract Surg 2015;31:2:124-8.