The retinal internal limiting membrane (ILM) forms the structural boundary between the retina and the vitreous. As a basement membrane, the ILM can act as a scaffold for cellular proliferation and is frequently involved in disorders that affect the vitreomacular interface, including the formation of epiretinal membranes (ERM), vitreomacular traction, and macular holes (MHs).
The ILM contains collagen fibrils, proteoglycans, basement membrane and the plasma membrane of Müller cells, and possibly other glial cells of the retina. Histologically, the ILM around MH also contains myofibrocytes. Contraction of these myofibrocytes has been suggested to both cause enlargement and prevent closure of MHs. Therefore, the removal of the ILM may be a surgical adjunct that can promote gliosis and the closure of MHs.
The removal of the ILM is considered difficult to perform because of the poor visibility of the ILM and the significant risk of inadvertent trauma to the retina. Difficult visualization of the ILM and firm attachment of the ILM to the underlying retina can present technical challenges when trying to peel this nearly invisible membrane, even for experienced vitreoretinal surgeons.
Indocyanine green dye has been used to stain the ILM and facilitate its removal. In the first study describing ICG-assisted ILM peeling in human cadaver eyes,1 researchers observed that ICG effectively stains the ILM after removal of the cortical vitreous. ICG staining greatly facilitated visualization of the ILM throughout the process of ILM peeling and clearly differentiated the edge between the stained ILM and unstained retina.
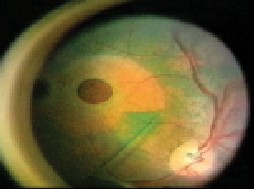 |
| Indocyanine green staining of the internal limiting membrane in bright green and the absence of staining of the underlying retina facilitates visualization of the ILM during macular hole surgery. |
ICG Pros
In another series, involving 13 eyes with stage 3 or stage 4 MH that underwent ICG-assisted ILM peeling, anatomical closure was achieved in 92 percent of patients, and a final visual acuity of 20/50 or better was achieved by 53.8 percent.3 Pathologic examination confirmed the presence of the ILM in nine eyes. Specimens were not processed from the remaining four eyes.
Other researchers reported a primary closure rate of 88 percent in 24 patients with stage 3 or 4 MH with ICG assisted ILM peeling, although the follow-up period was rather short (range 23 to 195 days); serum was also used and traumatic holes were included. Final visual acuity of 20/50 or better was achieved by 37.5 percent.4 These results were comparable to the rate of 42 percent (24 out of 57) reported in another study without any adjunct.6
In a 2003 report, 41 eyes with idiopathic stage 3 or 4 MH of median duration of 11 months achieved a primary anatomical closure rate of 89.7 percent with the use of ICG-assisted retinal ILM removal.5 In this study, the primary anatomical success rates for chronic and non-chronic MH were 78.9 and 95.5 percent, respectively. A final visual acuity of 20/50 or better was attained by 39 percent (16 eyes) of 41 eyes.5
An analysis of this prospective study and the 13-eye study mentioned above shows that the mean primary anatomical success rate of ICG-assisted ILM removal in idiopathic stage 3 or 4 MHs was 90.9 percent.3,5 These results were encouraging, as the mean primary anatomical closure rate of stage 3 or 4 MH without ILM peeling is about 70 percent. For series with ILM peeling without ICG staining, two other groups have reported rates of 53 and 56 percent.7,8
ICG-mediated ILM peeling has also been advocated for the surgical treatment of MH in pathologic myopia eyes. With the use of ICG to stain and peel the ILM, one group achieved a primary anatomical closure rate of 90 percent in a prospective study of MH in myopic eyes.9 This rate seems superior to the closure rates of 60 and 77 percent for MH surgery in high myopes reported in two other studies.10,11 Therefore, ILM peeling may have a role in MH surgery of severely myopic eyes by increasing the anatomical success rate.
Finally, ICG-assisted ILM peeling may be a valuable technique for the treatment of long-standing MH. A case report described a patient with long-standing (18 years) MH who underwent pars plana vitrectomy with peeling of the ILM after ICG staining.12 Postoperatively, the MH was closed, as confirmed by optical coherence tomography. Visual acuity increased from 20/200 to 20/50.
One study compared the rate of MH closure in patients treated in the standard fashion (group 1) versus those undergoing ILM peeling both without (group 2) and with (group 3) the use of ICG.13 In this series, both groups undergoing ILM peeling had a significantly improved rate of primary MH closure relative to traditional approaches (97 vs. 77 percent, with no difference seen between ILM peeling with and without ICG). There was, however, a statistically significant improvement in vision with the use of ILM peeling alone, but not when ICG was used as an adjuvant.
Potential retinal and retinal pigment epithelium damage related to ICG-assisted ILM peeling have been reported. In one study using ICG stain of the ILM in 20 patients,14 median postoperative best-corrected visual acuity was 20/200 (range, 20/500 to 20/40). Overall, there was no statistically significant improvement of BCVA. Visual field defects after the use of ICG occurred in seven of 20 patients.
Similar results were obtained in a recent prospective study comparing ILM peeling without (group 1) and with (group 2) ICG staining in 40 eyes with MH.15 In this series, the MH was closed in all patients. Visual acuity was improved in both groups, but after 12 months it was significantly better in the group not exposed to ICG (median, 0.85) as compared to the group in which ICG was used to facilitate ILM peeling (median, 0.60; P=0.02).
|
|
 |
|
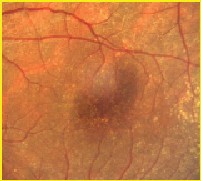
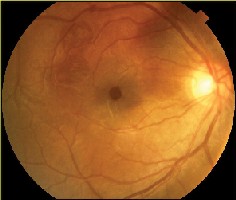
Figure 2A. Magnified clinical photo of the macula four months after MH repair with ICG-assisted ILM peeling. Note that there is no green stain of the retina visible clinically. |
Figure 2B. ICG auto-cyanescence photograph of the same eye shows persistence of ICG cyanescence from retinal pigment epithelium cells in the bed of the MH. The area where the ILM was peeled appears darker. |
|
|
|
 |
 |
 |
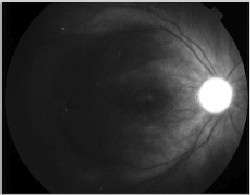 |
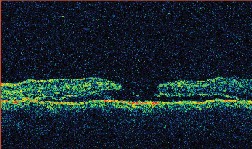
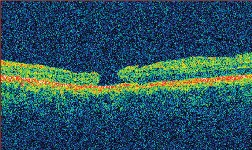
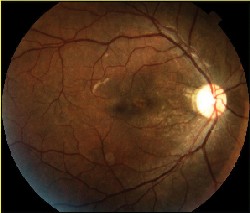
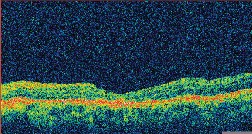
| Figure 3. A. (left to right) Clinical photograph of the right eye of a 17-year-old male, who presented with loss of vision to the 20/400 level after blunt trauma. There is a MH and subretinal hemorrhage at the posterior pole. B. Optical coherence tomography photograph of the same eye demonstrates the presence of a MH with a large cuff of subretinal fluid. C. OCT photo after surgery for MH repair without ILM peeling shows that the MH is still open, even if of reduced size. D. Clinical photo after repeated vitrectomy surgery with ICG-assisted ILM peeling demonstrates closure of the MH. There are RPE changes in the macula. Vision improved to 20/60. E. OCT confirms the complete closure of the MH. There are RPE changes in the fovea. F. ICG auto-cyanescence photograph demonstrates persistence of ICG signal in the optic disc. |
|
|
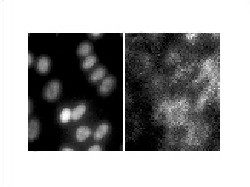
| Figure 4. A. Electron microscopy photograph of human RPE cells demonstrates the intracellular position of the RPE cells' nuclei, which appear white after staining with DAPI. B. Same RPE cells after incubation with ICG 5 mg/1 cc for one minute. There is selective intracellular localization of ICG molecules in the cytoplasm. |
Such a phototoxic effect associated with ICG staining of the RPE has been shown in experimental models. One group demonstrated an upregulation of apoptosis related genes, p53 and Bax, and cell cycle arrest protein, p21, in RPE cells treated with illumination and high doses of ICG.21
Other studies have shown that the concentration of ICG solution and the length of exposure may be a factor in determining RPE toxicity. RPE cells incubated with up to 5 mg/mL of ICG for five minutes or less exhibited no morphologic change and no significant decrease in dehydrogenase activity in one study.22 When RPE cells were exposed to 5 mg/mL of ICG for 10 minutes, 1 mg/mL of ICG for 20 minutes, or 0.01 mg/mL of ICG for three hours, cell morphologic features were altered, mitochondrial dehydrogenase activity decreased, and some cells were necrotic. The researchers concluded that exposure of RPE cells to ICG concentrations up to 5 mg/mL for five minutes or less was not injurious; prolonged exposure to a low ICG concentration was toxic. Since ICG may be retained in the vitreous cavity for a lengthy period, thorough washout of ICG during MH surgery should be required.
The dye has been found to remain in the eye for a mean (SD) of 2.7 (1.4) months after macular surgery, in contrast to the rapid clearance after ICG-capsulorhexis (6.0 [2.2] days).23 On the basis of these findings clinicians are trying to identify possible solutions to a safe use of ICG staining during vitreoretinal surgery.
Materials that may prevent the physical contact of RPE with the injected ICG—for example, high viscosity viscoelastics or perfluorocarbon heavy liquid—may limit RPE toxicity. Additionally, new vital stains will be soon available to assist the vitreoretinal surgeon in peeling the ILM.
Second Generation Vital Stains
The novel vital stains trypan blue (TB) and infracyanine green have been investigated clinically. Infracyanine green, which uses 5% glucose solution as its solvent for an iso-osmotic final solution, offers comparable staining of the ILM while reducing the untoward osmotic effects of ICG. In in vitro studies, infracyanine green in 5% glucose did not demonstrate cytotoxicity to cultured RPE cells, while ICG exposure led to significantly increased cell death.24
Similarly, in a clinical study, investigators found that ILM specimens excised with infracyanine green were not noted to include Müller cell footplates or other evidence of neural retinal disruption when examined by histopathology and electron microscopy.25
There is a large body of experience showing trypan blue, 0.06%, is apparently safe and useful for visualization of the anterior capsule during capsulorhexis in cataract surgery. At a concentration of 0.06%, trypan blue sometimes provides only faint staining of the ILM. For this reason, a multicentered study to evaluate the efficacy of trypan blue at a higher concentration of 0.2% for various indications in vitreoretinal surgery was set up.26 Fifty eyes of 50 patients with various vitreoretinal disorders were included in the study. Intraoperative trypan blue, 0.2%, was used in 22 cases with ERM, 18 cases with MH, two cases with a combination of MH and epimacular membrane, three cases with ILM and posterior hyaloid removal in diabetic retinopathy, and five cases with proliferative vitreoretinopathy.
A standard three-port pars plana vitrectomy was performed in all cases. After core vitrectomy was completed, posterior vitreous detachment was induced, and fluid-air exchange was performed. Then, 0.1 ml of trypan blue, 0.2% (MembraneBlue, DORC International, Zuidland, the Netherlands), was applied to the retina. After one minute, the dye was removed with a flute cannula (under air). A fluid-air exchange was then performed, and all excess dye was washed out. The stained ILM or ERM was removed using routine surgical techniques, usually by engaging the tissue with a pick or hooked needle, peeling the tissue from the retina, and removing it by using intraocular forceps.
Each surgeon was asked to score the visualization of the ILM or ERM after staining on a scale of none, poor, moderate, good, or excellent. A similar scale was used to subjectively assess the ease of removal of the membranes. For each eye, staining of the ILM or ERM and ease of removal was scored "good" or "excellent," except for one case. In the latter case, no ERM could be peeled off the macula. In all other cases, the ILM and/or ERM stained with a bluish color, whereas the underlying retina did not stain. The dye therefore facilitated visualization of the membrane's edges, their size and shape, and the extent of their excision. Generally, the ILM stained fainter than the ERM, but in all cases the ILM could be visualized. No side effects related to the intraoperative use of the dye were observed in any of the patients.
The introduction of trypan blue and infracyanine green by no means lays to rest the issue of retinal toxicity. As with ICG, with more study and clinical experience the limitations of trypan blue and infracyanine green will be defined while their dosages and application times are optimized. Other barriers also remain to be overcome, not the least of which is that trypan blue and infracyanine green are unavailable for surgical use in many countries. But we are moving beyond ICG as the sole dye to a second generation of vital stains for intraocular surgery.
ICG may be toxic to the RPE during MH surgery. Prolonged exposure of the RPE to light may enhance such toxicity. There is some evidence that ICG may also be toxic to the retinal ganglion cell and to the optic nerve. For the time being we recommend not using ICG for routine MH and epiretinal membrane surgeries. ICG may be helpful in performing a complete peel of the ILM in selected cases such as recurrent MH, traumatic MH and MH in pathologic myopia, especially when there is non optimal visibility of the retina during surgery. In such cases we advise using ICG solution at lower concentration, for brief exposure time, and avoiding prolonged illumination of the macular area with the endoillumunator probe.
Dr. Ciardella is in private practice at the Denver Health Medical Center, 777 Bannock St., MC 0156, Denver, Colo. 80204. Contact him at antonio.ciardel la@dhha.org.
Dr. Sparrow is a professor of ophthalmic science and pathology at Columbia University, 635 W. 165th St., Room 218, New York, NY 10032. Contact her at jrs88@columbia.edu. Dr. Chang is chair of the Department of Ophthalmology at Columbia University Medical School. Contact him at sc434@columbia.edu.
1. Burk SE, Da Mata AP, Snyder ME, Rosa RH Jr, Foster RE. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology 2000;107:2010-4.
2. Kwok AK, Lai TY, Yew DT, Li WW. Internal limiting membrane staining with various concentrations of indocyanine green dye under air in macular surgeries. Am J Ophthalmol 2003;136:223-30.
3. Kadonosono K, Itoh N, Uchio E, et al. Staining of internal limiting membrane in macular hole surgery. Arch Ophthalmol 2000;118:1116–8.
4. Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology 2001;108:1187–92.
5. Kwok AK, Lai TY, Chan W, et al. Indocyanine green staining and removal of retinal internal limiting membrane in stage 3 or 4 macular hole surgery. Br J Ophthalmol 2003;87:71–4.
6. Thompson JT, Smiddy WE, Williams GA, et al. Comparison of recombinant transforming growth factor-beta-2 and placebo as an adjunctive agent for macular hole surgery. Ophthalmology 1998;105:700–6.
7. Park DW, Sipperley JO, Sneed SR, et al. Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology 1999;106:1392–8.
8. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular hole surgery. Ophthalmology 2001;108:1471–8.
9. Kwok AK, Lai TY. Internal limiting membrane removal in macular hole surgery for severely myopic eyes: A case-control study. Br J Ophthalmol 2003;87:885-9.
10. Patel SC, Loo RH, Thompson JT, et al. Macular hole surgery in high myopia. Ophthalmology 2001;108:377–80
11. Sulkes DJ, Smiddy WE, Flynn HW, et al. Outcomes of macular hole surgery in severely myopic eyes: A case-control study. Am J Ophthalmol 2000;130:335–9.
12. Haritoglou C, Neubauer AS, Gandorfer A, et al. Indocyanine green for successful repair of a long-standing macular hole. Am J Ophthalmol 2003;136:389-91.
13. Sheidow TG, Blinder KJ, Holekamp N, et al. Outcome results in macular hole surgery: An evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology 2003;110:1697-701.
14. Haritoglou A, Gandorfer CA Gass MW, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: A clinicopathologic correlation. Am J Ophthalmol 2002;134:836–841.
15. Horio N, Horiguchi M. Effect on visual outcome after macular hole surgery when staining the internal limiting membrane with indocyanine green dye. Arch Ophthalmol 2004;122:992-6.
16. Weinberger A, Kirchhof B, Mazinani BE, et al. Persistent indocyanine green (ICG) fluorescence six weeks after intraocular ICG administration for macular hole surgery. Graefe's Arch Clin Exp Ophthalmol 2001;239:388–390.
17. Spaide RF. Persistent intraocular indocyanine green staining after macular hole surgery. Retina 2002;22:637-639.
18. Ciardella AP, Schiff W, Barile G, et al. Persistent indocyanine green fluorescence after vitrectomy for macular hole. Am J Ophthalmol 2003;136:174-7.
19. Tadayoni R, Paques M, Girmens JF, et al. Persistence of fundus fluorescence after use of indocyanine green for macular surgery. Ophthalmology 2003;110:604-608
20. Chang A, Morse LS, Handa JT, et al., Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 1998;105:1060–1068.
21. Yam HF, Kwok AK, Chan KP, et al. The effects of indocyanine green and illumination on gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2003;44;370–7.
22. Ho JD, Tsai RJ, Chen SN, Chen HC. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol 2003;135:258.
23. Horiguchi M, Nagata S, Yamamoto N, et al. Kinetics of indocyanine green dye after intraocular surgeries using indocyanine green staining. Arch Ophthalmol 2003;121:327-331.
24. Stalmans P, Van Aken EH, Veckeneer M, et al. Toxic effect of indocyanine green on retinal pigment epithelium related to osmotic effects of the solvent. Am J Ophthalmol 2002;134:282-285.
25. Stalmans P, Feron EJ, Parys-Van Ginderdeuren R, et al. Double vital staining using trypan blue and infracyanine green in macular pucker surgery. Br J Ophthalmol 2003;87:713-6.
26. Teba FA, Mohr A, Eckardt C, et al. Trypan blue staining in vitreoretinal surgery. Ophthalmology 2003;110:2409-12.