Ancillary imaging was obtained including fluorescein angiography (Figure 2) and optical coherence tomography of the right eye (Figure 3). IVFA revealed leakage from the optic disc of the right eye without macular leakage or vasculitis; a late frame of the left eye was normal. OCT demonstrated significant thickening of the retinal nerve fiber layer, macular edema in the outer nuclear layer with associated hard exudates in the outer plexiform layer (Figure 3, arrows) and subretinal fluid. The edema was primarily peripapillary and nasal to the fovea.
The differential diagnosis of this patient with sudden onset, unilateral vision loss due to optic disc edema and leakage with associated macular edema, subretinal fluid and hard exudates included infectious, inflammatory and neoplastic etiologies. Infectious causes included bacterial (e.g., Bartonella, tuberculosis, syphilis, Lyme disease), viral (e.g., mumps, herpes zoster), fungal (e.g., histoplasmosis), protozoan (e.g., toxoplasmosis) or nematodal (e.g., toxocara). Inflammatory etiologies included sarcoidosis, inflammatory bowel disease and polyarteritis nodosa. Leukemic infiltration of the optic nerve was the primary neoplastic consideration. Given the patient’s history of a deep cat scratch two months prior to presentation and her CT scan showing mesenteric adenitis, serologic testing for Bartonella henselae and Bartonella quintana was performed. Titers for B. henselae IgM and IgG were 1:40 and ≥1:1024, respectively; B. quintana titers were negative. Based on this, the patient was diagnosed with neuroretinitis secondary to cat-scratch disease and started on a 30-day course of doxycycline.
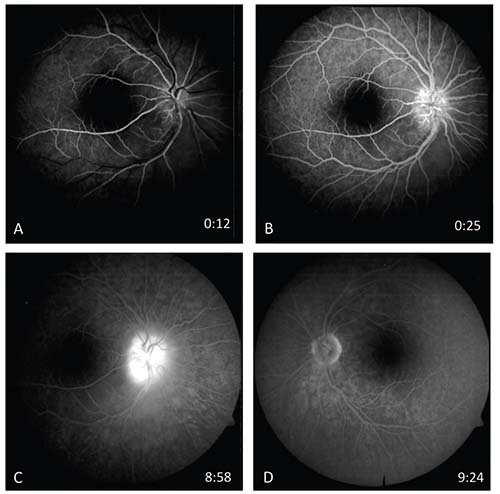 |
| Figure 2. Fluorescein angiography reveals leakage from the optic disc in the right eye (A-C) but no macular leakage or vasculitis. A late frame of the left eye (D) is normal. |
Discussion
Bartonella species are facultative intracellular, slow-growing gram negative rods that typically reside in erythrocytes or endothelial cells to evade the host’s immune response.1,2 Cat-scratch disease (CSD) is caused by the bacterium B. henselae, which is maintained and spread among cats via the cat flea and transmitted to humans through scratches, and possibly bites, from cats.3 In the United States, the incidence is highest in the south Atlantic and south central states, although the disease occurs throughout the country and worldwide.4 Almost one-third of cases occur in children 14 years of age or younger, with the highest annual incidence in children 5 to 9 years old. Women are more commonly affected than men, and the peak incidence occurs in January, followed by August through November.3
The manifestations of CSD can be described as typical (85 to 90 percent) or atypical (10 to 15 percent) disease. Typical disease consists of a painless
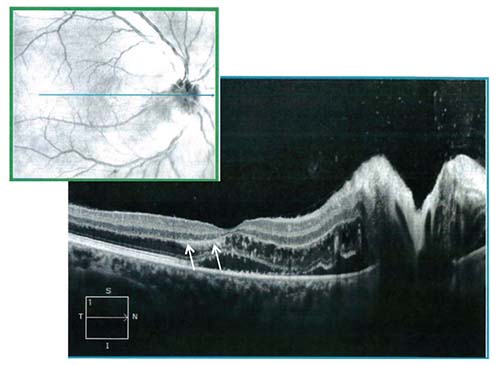 |
| Figure 3. OCT through the macula and optic nerve of the right eye demonstrating significant thickening of the retinal nerve fiber layer, macular edema in the outer nuclear layer with hard exudates in the outer plexiform layer (arrows), and subretinal fluid. |
Ophthalmologic involvement in CSD is estimated to occur in 5 to 10 percent of patients, with Parinaud oculoglandular syndrome the most common manifestation, occurring in 2 to 5 percent.2,5 Neuroretinitis, although a relatively rare complication of CSD, is one of the most recognizable. One study found that 64.3 percent of patients with a clinical diagnosis of neuroretinitis had elevated B. henselae IgM or IgG on serology.6 In comparison, only 3 percent of healthy individuals are seropositive for B. henselae.7
Neuroretinitis was first described by Theodor Leber in 1916, which he described as a “stellate maculopathy.”8 Gass subsequently used FA to demonstrate that the site of leakage is the optic nerve, not the macula, and he suggested changing the name to “neuroretinitis.”9 The typical presentation is sudden-onset unilateral vision loss associated with optic disc edema, macular edema and serous retinal detachment. A macular star pattern often develops two to four weeks after onset as the aqueous phase of the exudate is resorbed and lipid remains in the outer plexiform layer.10,11 In two cohorts of patients with either CSD neuroretinitis or optic neuropathy (optic disc edema with or without macular involvement), 14.5 to 37.7 percent of eyes had an initial visual acuity of 20/40 or better, 22.6 to 33.3 percent were between 20/50 and 20/200, and 39.6 to 52.2 percent had worse than 20/200 visual acuity. Both studies found that the majority of patients achieved a visual acuity outcome of 20/40 or better, and that a relative afferent pupillary defect was present in most unilateral cases, but its absence was noted in some instances of severe vision loss, possibly due to a greater contribution from macular disease. Vitritis was present in only 13 percent of eyes.8,12
Although most commonly a unilateral disease, the case presented here demonstrates that CSD can present with bilateral but markedly asymmetric ocular findings. One series reported that 4/65 cases (6 percent) of CSD neuroretinitis were bilateral, while another found bilateral neuroretinitis in 2/26 cases (8 percent).8,12 In the latter study, however, when considering the entire cohort of patients with CSD optic neuropathy, 9/53 cases (17 percent) demonstrated bilateral disease. The prevalence may be even higher if a broader spectrum of ophthalmic manifestations in individuals who are seropositive for CSD is included, with one study finding bilateral intraocular changes in 13/24 patients (54.1 percent) who were positive for B. henselae IgG.13
Numerous posterior segment findings in CSD have been reported, including focal or multifocal chorioretinitis,8,14 intermediate uveitis and vasculitis,15 retinal artery occlusion,16 retinal vein occlusion,17 macular hole18 and choroidal neovascularization.19 Interestingly, the protean manifestations of different Bartonella species not only depend on the particular organism involved but also the strength of the host’s immune system.2 Bacillary angiomatosis, a well-known entity caused by B. henselae in immunocompromised patients, reflects Bartonella’s ability to trigger a vasoproliferative response in these individuals. Similarly, a case report of three HIV-positive men with positive B. henselae serologies revealed that all presented with yellowish subretinal lesions that displayed abnormal vascular networks on FA.20 The diverse manifestations of B. henselae necessitate maintaining a high index of suspicion for it in the work-up of these patients.
Indirect fluorescence assay (IFA) and enzyme immunosorbent assay (EIA)21 are used to assess for serologic diagnosis of B. henselae. IFA is the commercially available serologic test, and at an IgG titer cutoff of 1:64, one study found the test to be 88-percent sensitive and 94-percent specific.7 In our patient, the titer was ≥1:1024, which has almost 100-percent specificity for diagnosing B. henselae. Sensitivities and specificities are lower in HIV-positive patients,22 and cross-reactivity can occur with B. quintana,2 as well as with Chlamydia and Coxiella burnetii. Polymerase chain reaction-based testing on tissue or blood is also available and is very specific but sensitivity is suboptimal.4 Finally, B. henselae can be cultured but tends to be very slow growing, given its fastidious nature.
Because the clinical history in our patient was so strongly suggestive of CSD, no further work-up was needed, but an MRI might be obtained in the evaluation of similar clinical presentations. CSD optic neuropathy has demonstrated enhancement of a 3- to 4- mm segment at the optic nerve-globe junction, whereas other etiologies for optic neuropathy often involve a longer segment of optic nerve that’s more posterior to the globe.23 While nearly half of CSD patients have normal MRI findings, the described pattern of optic nerve enhancement may suggest B. henselae as the underlying etiology.
CSD is a self-limited illness, so it is difficult to draw conclusions regarding the efficacy of treatment based on published case reports given the possibility for spontaneous visual recovery. In general, antibiotics are initiated for immunocompetent patients with atypical disease and those who are immunocompromised, with immunocompromised patients receiving a longer course of treatment.2,24 Doxycycline, with or without rifampin, and erythromycin have been commonly used; ciprofloxacin and trimethoprim-sulfamethoxazole are other frequently administered antibiotics.8 Based on current available data, no conclusions can be drawn about the utility of steroids.
In conclusion, cat-scratch disease secondary to Bartonella henselae should remain a strong consideration in a patient presenting with neuroretinitis, especially with the proper clinical context, although numerous infectious and inflammatory etiologies must be considered in the differential diagnosis. Likewise, B. henselae can produce diverse ophthalmic manifestations beyond neuroretinitis, requiring a high index of suspicion to make the proper diagnosis. REVIEW
1. Giladi M, Ephros M. Harrison’s Principles of Internal Medicine. 19th ed. New York: McGraw-Hill, 2014. Chapter 197, Bartonella Infections, Including Cat-Scratch Disease. http://accessmedicine.mhmedical.com.
2. Roe RH, Jumper JM, Fu AD, et al. Ocular bartonella infections. Int Ophthalmol Clin 2008;48:93-105.
3. Nelson CA, Saha S, Mead PS. Cat-scratch disease in the United States, 2005-2013. Emerg Infect Dis 2016;22:1741-46.
4. Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat-scratch disease: Widening spectrum of Bartonella henselae infection. Pediatrics 2008;121:e1413-25.
5. Carithers HA. Cat-scratch Disease. An overview based on a study of 1,200 patients. Am J Dis Child 1985;139:1124-1133.
6. Suhler EB, Lauer AK, Rosenbaum JT. Prevalence of serologic evidence of cat scratch disease in patients with neuroretinitis. Ophthalmology 2000;107:871-876.
7. Regnery RL, Olson JG, Perkins BA, et al. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 1992;339:1443-5.
8. Purvin V, Sundaram S, Kawasaki A. Neuroretinitis: Review of the literature and new observations. J Neuro-Ophthalmol 2011;31:58-68.
9. Gass JDM. Diseases of the optic nerve that may simulate macular disease. Trans Am Acad Ophthalmol Otolaryngol 1977;83:766-769.
10. Ghauri RR, Lee AG. Optic disk edema with a macular star. Surv Ophthalmol 1998;43:270-274.
11. Wade NK, Levi L, Jones MR, et al. Optic disk edema associated with peripapillary serous retinal detachment: An early sign of systemic Bartonella henselae infection. Am J Ophthalmol 2000;130:327-334.
12. Chi SL, Stinnett S, Eggenberger E, et al. Clinical characteristics in 53 patients with cat scratch optic neuropathy. Ophthalmology 2012;119:183-187.
13. Curi ALL, Machado D, Heringer G, et al. Cat-scratch disease: Ocular manifestations and visual outcome. Int Ophthalmol 2010;30:553-58.
14. Pichi F, Srivastava SK, Levinson A, et al. A focal chorioretinal Bartonella lesion analyzed by optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging 2016;47:585-88.
15. Soheilian A, Markomichelakis N, Foster CS. Intermediate uveitis and retinal vasculitis as manifestations of cat scratch disease. Am J Ophthalmol 1996;122:582-584.
16. Eiger-Moscovich M, Amer Ragonde, Oray Merih, et al. Retinal artery occlusion due to Bartonella henselae infection: A case series. Acta Ophthalmol 2016;94:e367-370.
17. Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol 1999;10:209-216.
18. Albini TA, Lakhanpal RR, Foroozan R, et al. Macular hole in cat scratch disease. Am J Ophthalmol 2005;140:149-151.
19. Latanza L, Viscogliosi F, Solimeo A, et al. Choroidal neovascularisation as an unusual ophthalmic manifestation of cat-scratch disease in an 8-year-old girl. Int Ophthalmol 2015;35:709-716.
20. Curi ALL, Machado DO, Heringer G, et al. Ocular manifestations of cat-scratch disease in HIV-positive patients. Am J Ophthalmol 2006;141:400-401.
21. Szelc-Kelly CM, Goral S, Perez-Perez GI, et al. Serologic responses to Bartonella and Afipia antigens in patients with cat scratch disease. Pediatrics 1995;96:1137-42.
22. Dalton MJ, Robinson LE, Cooper J, et al. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med 1995;155:1670-76.
23. Schmalfuss IM, Dean CW, Sistrom C, et al. Optic neuropathy secondary to cat scratch disease: Distinguishing MR imaging features from other types of optic neuropathies. Am J Neuroradiol 2005;26:1310-1316.
24. Biancardi AL, Curi ALL. Cat-scratch disease. Ocular Immunology and Inflammation 2014;148-154.



