There’s no question that anti-VEGF drugs have caused a sea-change in the treatment of several retinal diseases. Conditions that were basically untreatable have now become manageable, preventing what was once almost inevitable blindness. Yet there’s still plenty of room for improvement; results vary, and not all patients avoid vision loss.
“When I started my career, neovascular age-related macular degeneration was a horrible disease,” recalls K. Bailey Freund, MD, a retina specialist and a clinical professor of ophthalmology at the New York University School of Medicine. (Dr. Freund has been a principal investigator in several pivotal trials of novel treatments for retinal diseases.) “Nothing we could do would really help our patients. Today, certain patients do extremely well—particularly the ones that have Type 1 (subretinal pigment epithelium) neovascularization under the fovea. Others do well initially, but eventually the non-neovascular aspect of the disease kicks in and they ultimately end up losing vision, more from macular atrophy than exudative complications.”
Here, retina specialists answer key questions about the current state of anti-VEGF drug treatments for macular degeneration and other retinal diseases; share the latest insights, which suggest that neovascularization and subretinal fluid are not always a bad thing; and discuss what the upcoming years may hold.
Anti-VEGF Drug Choice
“Each anti-VEGF option has an advantage that certain people prefer,” notes Philip Rosenfeld, MD, PhD, professor of ophthalmology at the Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine. “For example, bevacizumab is cheap. In certain countries surgeons can get a rebate for using ranibizumab, so some doctors use it because it’s financially rewarding for the practice. Aflibercept is the preferred drug for certain neovascular types of disease; for example, I think it works best in fibrovascular retinal pigment epithelial detachments with a significant serous component.
“Personally, I treat with bevacizumab and aflibercept,” he says. “In my non-hospital-based setting, I always start with bevacizumab and try to get a durability that exceeds six, eight or 10 weeks. If I can’t get beyond that six-to-eight week interval, then I switch to aflibercept. Some surgeons prefer ranibizumab, and others prefer to use aflibercept exclusively. However, many times when patients are in Medicare Advantage plans, the choice of drug is decided by the provider.”
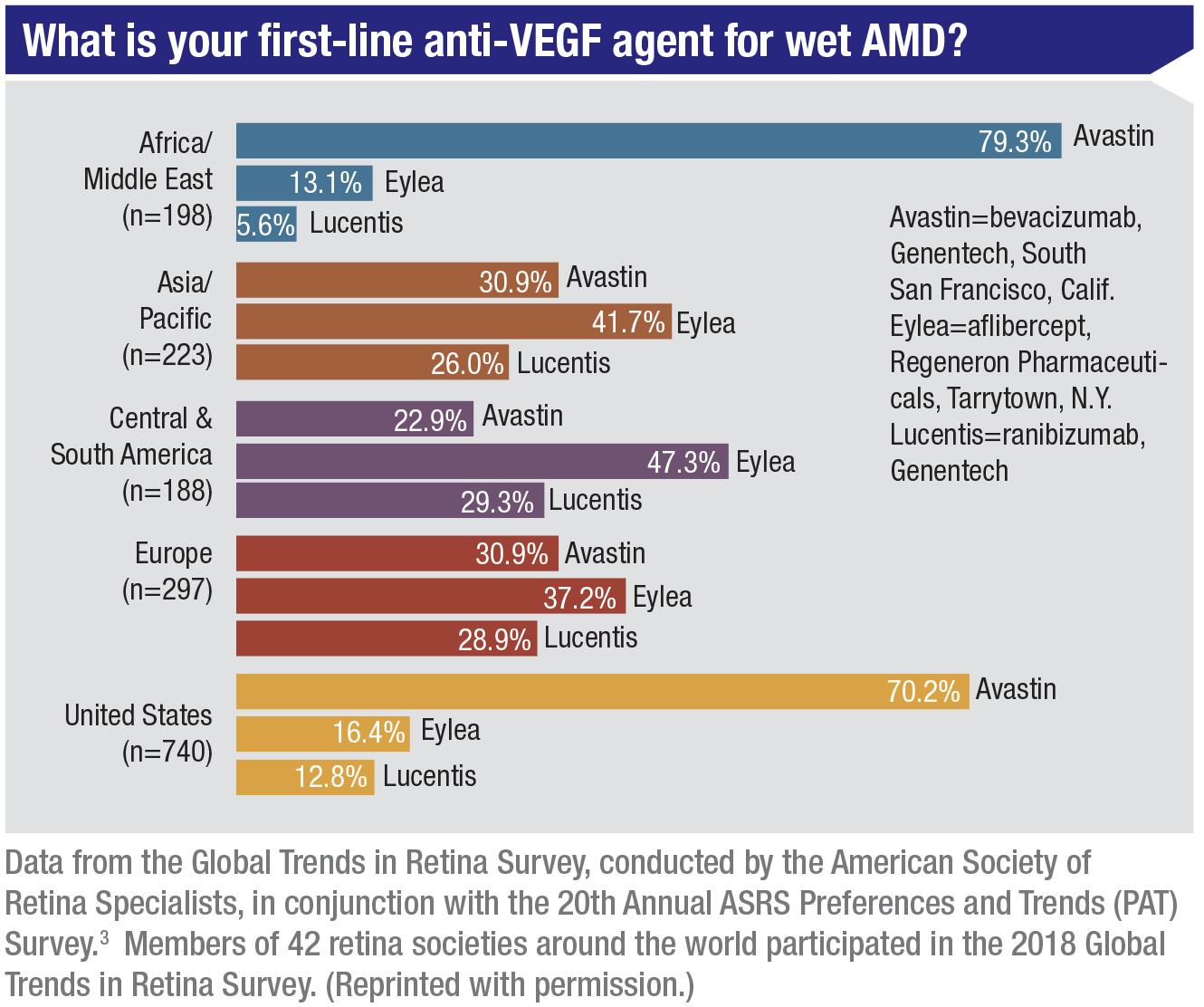 |
Dr. Rosenfeld notes that The American Society of Retina Specialists runs an annual Patterns and Trends survey, in which they ask about anti-VEGF drug choices every year. “We don’t have the results for 2019 as we speak,” he says, “but the replies for 2018 indicated that in the U.S., bevacizumab is the first-line choice for treating wet AMD for about 70 percent of retina specialists. Ranibizumab is first-line for 12 percent, and aflibercept is first-line for 16 percent. Outside the U.S.—except in Africa and the Middle East—it looks like aflibercept wins. However, treating physicians are litigating for access to bevacizumab in the U.K. In France, it’s still very hard to get.” (He notes that at a recent retina meeting in Italy, most treating physicians said that they’ve used all three drug options—ranibizumab, bevacizumab and aflibercept.)
Dr. Freund says he finds the current anti-VEGF agents to be fairly similar in terms of efficacy and safety, so he chooses his drug regimen based on the individual patient’s presentation. “I might choose one drug over another when a patient is somewhat refractory to treatment,” he says. “This is particularly true for eyes that have what I currently refer to as aneurysmal Type 1 neovascularization, more commonly known as polypoidal choroidal vasculopathy. I see many patients with this lesion growth pattern because it was first described in our practice, and I’ve published on it extensively.
“Eyes with this lesion growth pattern may have a more robust response to aflibercept,” he continues. “Clinical trial evidence from the PLANET study showed that many of these eyes respond well to aflibercept monotherapy, while data from the EVEREST II trial indicates that eyes with aneurysmal Type 1 neovascularization receiving ranibizumab may require the addition of photodynamic therapy in order to optimize visual outcomes.
“There’s another form of neovascular AMD that I call pachychoroid neovasculopathy,” he notes. “These eyes have choroidal findings that are similar to those seen in eyes with central serous chorioretinopathy. Also, they lack some of the characteristic clinical findings we associate with typical macular degeneration in elderly Caucasian patients, such as soft drusen. Those eyes may benefit from aflibercept, since aflibercept has been shown to decrease choroidal thickness more than the other agents. I find that eyes which are somewhat refractory to anti-VEGF therapy may not respond as well to bevacizumab, compared to the other agents.
 |
“In the final analysis, a typical patient responds well to all of these agents,” he says. “Numerous studies show that bevacizumab is noninferior to the alternative FDA-approved options. However, for patients managed on a treat-and-extend regimen, I’m less comfortable extending my dosing interval beyond eight weeks with bevacizumab. So, for patients with no out-of-pocket drug expense, we may choose a different agent that could require fewer injections, particularly when frequent office visits would be very difficult.”
Of course, some patients don’t respond to the first drug the treating physician tries, but Dr. Freund points out that switching refractory patients to an alternative anti-VEGF is not a cure-all. “When aflibercept was approved, many practices did what we then called ‘switcher’ studies,” notes Dr. Freund. “We took patients who were poorly controlled with monthly bevacizumab or ranibizumab and switched them to aflibercept, expecting that because most eyes in the aflibercept trials could be maintained on injections every eight weeks, the same might happen with these refractory cases. But the eyes we were switching were very different from the type of newly-diagnosed cases enrolled in clinical trials. All we typically found was that aflibercept might get rid of more fluid for a little longer than the prior agents. We did not find that eyes showing persistent fluid with monthly ranibizumab were fluid-free for eight weeks with aflibercept.”
Regarding the treatment protocol, Dr. Rosenfeld says he believes that most clinicians treat their patients using a treat-and-extend or modified treat-and-extend regimen. “This usually means performing monthly dosing until the macular fluid is resolved, and then extending the interval until macular fluid recurs,” he explains. “At that point the interval is shortened, and eventually a treatment interval is defined for that particular patient. When I attended the FLORetina meeting in Florence, Italy recently, this protocol seemed to be the general consensus among the attendees.”
Is Neovascularization Bad?
Clearly, neovascularization can be part of the problem in retinal diseases such as macular degeneration. However, many researchers note that anti-VEGF drugs aren’t really treating the neovascularization—and they’re beginning to suspect that neovascularization may actually be a good thing in some patients.
“There are three types of macular neovascularization,” Dr. Rosenfeld explains. “The major type is what’s called Type 1, which occurs under the retinal pigment epithelium. Today, with OCTA, particularly swept-source OCTA, we can identify Type 1 neovascularization long before exudation occurs. For that reason there’s been some debate about whether we should treat Type 1 lesions with anti-VEGF therapy before exudation starts.
“I think the consensus is that we should not,” he says. “Instead, we should watch the lesions and treat when symptomatic exudation develops. The reason is that those lesions don’t go away because of treatment. What we’re doing is training them not to leak. We calm the lesion down, but we don’t make it go away.
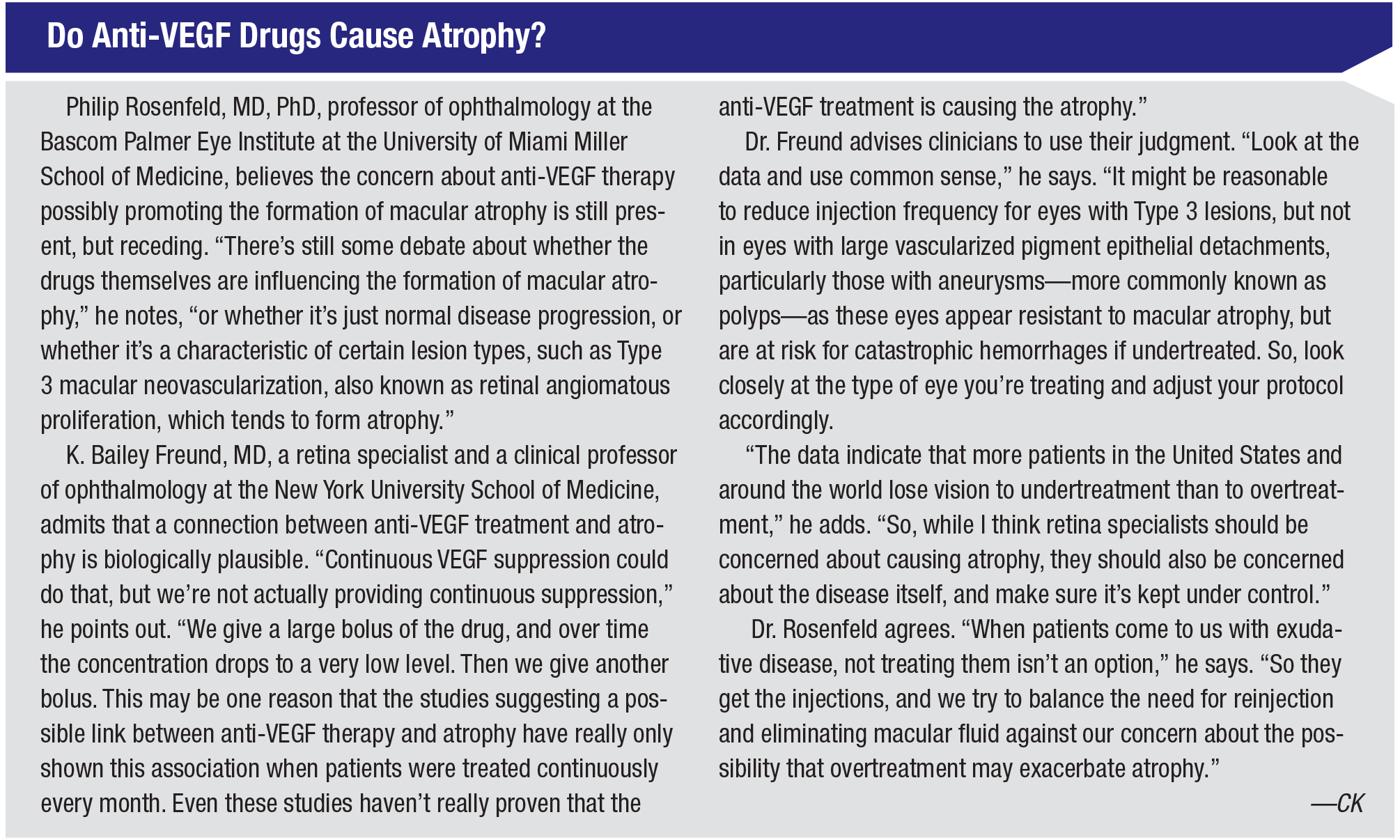 |
“In fact, we’re starting to believe that when the Type 1 lesions go away, you’re left with macular atrophy,” he says. “Many of my colleagues believe that you need to manage the neovascularization, but you don’t want to overtreat it. You want the neovascularization to stay there and just stop leaking. To put it another way, the field has evolved to believe that neovascularization is not the enemy; exudation and hemorrhage are the enemy. The neovascularization may actually be serving a very important function—providing nutritional support for the retinal pigment epithelium. Its appearance may be a normal physiologic response to a pathological condition in which you’re losing the choriocapillaris and there’s low-grade inflammation in the back of the eye. You need this neovascular complex. You just don’t want the exudation.”
“We know that patients that look clinically dry can harbor ‘non-exudative’ Type 1 neovascular tissue, which proliferates beneath the RPE with little clinical or optical coherence tomography evidence of its presence,” says Dr. Freund. “There’s no fluid and no hemorrhage. Today, however, we have tools like OCTA that let us detect these vessels. The most notable fact is that some of these patients never develop pathologic exudation. The challenge is that we don’t know exactly what percentage of those patients will eventually develop vision-threatening fluid and hemorrhage.”
Dr. Freund frequently collaborates with Christine A. Curcio, PhD, a professor of ophthalmology at the University of Alabama at Birmingham, who is an expert in the histology of macular degeneration. “We’ve been performing clinicopathologic correlations for AMD for close to a decade,” he says. “If patients from my practice consent, we enroll them in our donor program, and the eye bank recovers the whole globe following their death. Our mission is to correlate the histology with the retinal imaging done in the clinic.
“We’re currently studying the eyes of a patient followed for 10 years,” he says. “When she first presented, she was in her late 70s, and she’d already lost central vision in one eye from scarring due to neovascular AMD. Surprisingly, even though fluorescein angiography and OCT documented Type 1 neovascularization in her other eye, this eye never developed sight-threating exudation, and retained excellent visual acuity for more than a decade. This unusual disease course was documented with multimodal retinal imaging, which we’re now correlating with high-resolution histology. Unexpectedly, it turns out that more than half of her macula was sitting on top of neovascular tissue, and the overlying photoreceptors maintained a healthy structure. The neovascular tissue appears to have maintained nutritional support to the fovea as the patient’s native choriocapillaris failed due to aging. So, by the time she died, the health of her outer retina appears to have been maintained by the very same tissue that we all fear is going to cause patients to lose vision—tissue that we often do everything in our power to suppress with our anti-VEGF agents.”
Dr. Freund notes that the idea that Type 1 neovascularization could be beneficial isn’t new. “This idea was first proposed by Hans E. Grossniklaus, MD and Richard Green, MD,” says Dr. Freund. “They hypothesized that Type 1 neovascularization had the potential to recapitulate the normal choriocapillaris, and this concept appears to be supported by the findings in our current donor. In some patients, these non-exudative vessels may actually be helping to preserve the overlying retina. So, the goal may not be to destroy or completely inhibit their growth but to carefully monitor eyes with these vessels so that pathologic exudation can be caught and treated early.
“That means we need to have the right balance,” he concludes. “We shouldn’t be so aggressive that we destroy a potentially protective mechanism that could benefit our patients in future, but we also shouldn’t let it get out of control and damage vision quickly with catastrophic bleeding.”
Is Fluid Always a Bad Thing?
“Studies have shown that eyes treated with intravitreal anti-VEGF therapy which continue to manifest subretinal fluid—eyes that don’t go completely dry—may do as well, or even better, than eyes which dry up completely,” notes Dr. Freund. “So, a little bit of fluid may not be such a bad thing.”
Dr. Freund refers to the three main types of neovascularization occurring in exudative AMD, and says evidence is mounting that they may warrant different treatment regimens. “I believe retinal specialists should look at the eye’s presenting imaging characteristics, which define a lesion’s subtype,” he says. “Different subtypes seem to respond differently to treatment and call for different levels of monitoring. For example, it’s my strong belief that eyes presenting with Type 1 neovascularization beneath the fovea that continue to show a small amount of subretinal fluid, despite frequent dosing, are more resistant to geographic atrophy than eyes with other neovascular lesion patterns. In these eyes, a little bit of fluid is probably not such a bad thing. The main threat to vision in eyes like this appears to be the occurrence of subretinal hemorrhage if treatment is discontinued.
“In contrast, atrophy is a greater concern in eyes that have intraretinal neovascularization known as a Type 3 neovascularization or RAP (retinal angiomatous proliferation) pattern,” he continues. “Greater susceptibility to macular atrophy in eyes with Type 3 lesions has been observed in several large clinical trials. Patients presenting with Type 3 neovascularization are often older individuals with thin choroids and focal areas of macular hyperpigmentation, both of which increase susceptibility to macular atrophy. For eyes presenting with Type 3 neo-vascularization, I’ll often first try PRN dosing, for three reasons: lesion quiescence may be long-lasting; recurrences predictably occur at the initial site of activity; and recurrent exudation with Type 3 lesions is rarely accompanied by large hemorrhages.”
 |
Dr. Freund notes that Type 1 and Type 3 are the two most common variants of neovascular AMD. “Type 2 and mixed lesions with a component of Type 2 are less common, but the vessels in these eyes have broken through the RPE to proliferate in the subretinal space,” he says. “You have to be careful with these cases, since recurrent exudation from Type 2 lesions is in direct contact with the vulnerable photoreceptors. In that situation irreversible vision loss can occur quickly.”
Dr. Freund points out that the implication is that different types of lesions should be treated differently. “Don’t simply assume that all neovascular AMD is the same and should be treated with the same regimen,” he says. “Over the short term, these slight variations in regimen may not seem to make a difference, but we often treat patients for the rest of their lives. Little differences in how a patient does on one regimen versus another end up making a big difference over five, 10 or 15 years of treatment.”
Dr. Freund says that OCT may provide clues to the best treatment protocol. “If you see a shallow, irregular PED with heterogeneous internal contents in a patient with other findings of AMD, there’s a high probability that this finding represents neovascular tissue,” he says. “If there’s no exudation, eyes with this finding should be monitored closely, and the patient should be instructed to use an Amsler grid to monitor for conversion to active wet disease.
“When this type of vascularized pigment epithelial detachment, or Type 1 pattern, is subfoveal and associated with exudation, I use a treat-and-extend regimen of anti-VEGF therapy to control exudation,” he continues. “However, my goal is not to flatten the PED itself. I believe that intense treatment has the potential to convert a vascularized PED into avascular fibrotic tissue—tissue that will no longer be able to support the overlying retina. If there’s no hemorrhage, I’m not concerned about a little bit of persistent subretinal fluid, as long as it’s not increasing over time.”
Dr. Freund says that many of his patients have demonstrated the wisdom of this approach. “I have many patients I’ve been treating with anti-VEGF therapy for 10 or more years, some of whom have had as many as 100 injections,” he says. “Many eyes still have close to 20/20 vision.”
How Important is OCTA?
“Right now we treat based on exudation—the fluid that’s visible on structural OCT,” notes Dr. Rosenfeld. “However, people are working hard to see if the information we pick up with OCTA may be able to influence how we treat. Many papers have been published about using OCTA to look at different types of neovascular structures that exist in these eyes with wet AMD, hoping that some of what they find would be predictive of how often we need to treat, and/or the outcome of treatment. The results have been underwhelming, which is probably because the anti-VEGF drugs treat the exudation, not the neovascularization. Thus, changes in the vasculature revealed by OCTA may be less important than the presence of exudation on structural OCT.
“However, OCTA does have a role,” he says. “It’s a very powerful way to identify eyes with dry macular degeneration that are at higher risk of exudation, so we can follow them differently. With OCTA—in particular SS-OCTA—you can see the neovascularization many months before patients actually develop exudation. It’s growing silently. This has been known since the 1970s, when autopsy eyes with dry macular degeneration were shown to harbor neovascularization; it just wasn’t leaking or bleeding. Using ICG angiography in the 1990s, retina specialists also showed that this neovascularization existed in their living patients. However, we couldn’t routinely screen patients with ICG angiography, so this discovery was largely forgotten until OCTA came along.
“Knowing that this neovascularization is present, via OCTA, is valuable information,” he says. “We recently conducted a two-year study involving 227 patients, which is currently in press. We compared eyes with and without this nonexudative neovascularization and found that there’s a 14-fold increased risk of exudation over a period of two years when neovascularization is present. So OCTA serves a purpose—just not for monitoring lesions once you start treating. Instead, it can help to identify these lesions before exudation develops.
“For this reason, I use OCTA to check all of my dry AMD patients to see if they have these lesions,” he says. “It changes how I manage my patients. If they have the lesions, I usually follow them every two months. If they don’t have the lesions, I usually follow them every six months. It also helps me educate patients so they understand what’s going on and become partners in their own management.”
Dr. Freund says he uses OCTA frequently, but doesn’t believe it’s essential for diagnosing or treating AMD. “Often, OCT alone is sufficient to diagnose neovascular AMD or to arouse high suspicion that there’s a problem,” he notes. “In some cases, when there’s a characteristic OCT pattern of specific neovascular features, dye angiography may not be needed, especially if there’s a hemorrhage. I think OCTA is helpful if, like myself, you’re interested in identifying the neovascular lesion type to help individualize your treatment algorithm.
“Does individualizing the treatment regimen necessarily lead to better outcomes?” he adds. “I think so, but this remains to be proven. For now, I’d say that OCTA is a valuable research tool. In the future, it may become essential for a clinician managing macular degeneration.”
Dr. Rosenfeld believes clinicians will benefit from using OCTA. “I think everyone who practices retinal care needs access to OCTA, whether it’s for diabetes or AMD or vein occlusions,” he says. “It’s a remarkable tool that allows us to see the full extent of the underlying disease, and in certain situations helps us manage it better. OCTA is now our number one tool for diagnosing proliferative diabetic retinopathy. It helps us determine the extent of vascular nonperfusion in diabetics and vein occlusion, and it’s particularly useful for identifying the subclinical, nonexudative lesions in dry AMD. Furthermore, patients love it compared with dye-based angiograms. Meanwhile, of course, structural OCT is very useful for managing all of these conditions as well. But with OCTA scans, you get both the structural OCT and the angiographic OCT.”
Dr. Rosenfeld points out one big problem with OCTA, however, at least in the United States. “We don’t have a unique billing code for OCTA,” he explains. “The scan time is about the same as routine OCT, but the time it takes for the physician to interact with the instrument, look at the scans and extract the information by manipulating the segmentation boundaries to get the best possible image isn’t currently reimbursed. So, while the equipment is more expensive and it takes more time to evaluate the scans, the reimbursement is the same as for the more typical OCT. Outside the U.S., OCTA has a higher reimbursement, so the technology has become very popular. But in the U.S., it’s a difficult product to sell, because doctors want the revenue from dye-based angiography.
“Despite that concern, I believe it’s worth using OCTA,” he concludes. “With it, you achieve increased patient satisfaction and patient wait time is decreased. You can see more patients, because dye-based angiography takes a long time to perform—and it’s risky. So from an economic standpoint I think OCTA wins, even without the unique billing code.”
 |
What’s Next?
As effective as the current options are, new drugs and technology in the pipeline have surgeons hoping for even better outcomes—and a reduced injection burden.
• Brolucizumab. This is an investigational anti-VEGF drug from Novartis. “We hope it will provide a greater duration of action,” Dr. Rosenfeld explains.
Dr. Freund notes that brolucizumab was compared head to head with aflibercept in a trial. “The trial compared the labeled dosing of Eylea—every four weeks times three, extended to once every eight weeks—to brolucizumab, which is given every four weeks for three injections and then extended to every 12 weeks,” he explains. “With brolucizumab, a little more than half of the eyes could be maintained on the 12-week dosing to the final 48-week endpoint. Unfortunately, the trial design makes it hard to do a direct comparison between the drugs because patients treated with aflibercept were never extended beyond eight weeks. However, during the first three months, all the eyes were dosed every four weeks. During that period, there was more improvement in OCT thickness with brolucizumab than with aflibercept. “Part of the reason for this finding is that brolucizumab is a smaller molecule in a more concentrated formulation,” he points out. “When you inject the same volume of both drugs, the molar concentration of brolucizumab is approximately 10 times that of aflibercept. That means you’re getting a lot more drug in the eye with each injection of brolucizumab.”
One reason surgeons look forward to new options is the possibility that they’ll help to treat refractory patients. However, Dr. Freund points out that it could be problematic to use brolucizumab for this purpose, at least at the outset, because of the trial design. “Let’s say you have a patient that doesn’t dry up as much as you hope using one of the other agents,” he says. “In the trials of the other drugs there were arms in which patients were treated monthly to the endpoint. Brolucizumab didn’t have that, so it seems unlikely that the initial approval will include long-term monthly dosing. The reality is, if a refractory patient can’t go for four or five weeks on the other agents, they’ll probably have trouble going for eight or 12 weeks with brolucizumab. That may limit how much you can use that drug for these difficult, refractory patients.
“I believe the company is doing other studies that may eventually get a label allowing monthly treatment,” he adds, “but at the launch that could be a bit of an issue.”
• Abicipar pegol. Another drug being tested is Allergan’s abicipar pegol. “The trial design was basically 12-month dosing head-to-head between abicipar pegol and monthly ranibizumab,” Dr. Freund explains. “The new drug met its noninferiority endpoint, which is fairly impressive. Patients went to 12 weeks and seemed to do just as well as patients getting ranibizumab every month.
“The issue is that there’s been some inflammation with abicipar pegol,” he continues. “Initially about 15 percent of patients had inflammation. However, the company subsequently released data from a trial called MAPLE, where a change in the manufacturing of the drug reduced the inflammation rate to 8 or 9 percent, and so far, there’s no evidence that the inflammation caused in any of these patients was of much concern. So, you could decide to try abicipar pegol on your patient once. If the patient develops inflammation, you could stop. The risk/benefit ratio would be pretty good: There may be only a 9-percent chance that that the patient will have inflammation, but there’s close to a 90-percent chance that the patient will be able to be dosed once every 12 weeks. With brolucizumab you’d only have a 50-50 chance of reaching every 12 weeks. That’s one way to look at the data.”
• Sustained delivery. A key way to reduce the injection burden, of course, is via sustained delivery. “No one likes injections,” notes Dr. Freund. “But now Genentech has an implantable port that’s a refillable reservoir. Theoretically, you’d only have to do two refills a year.”
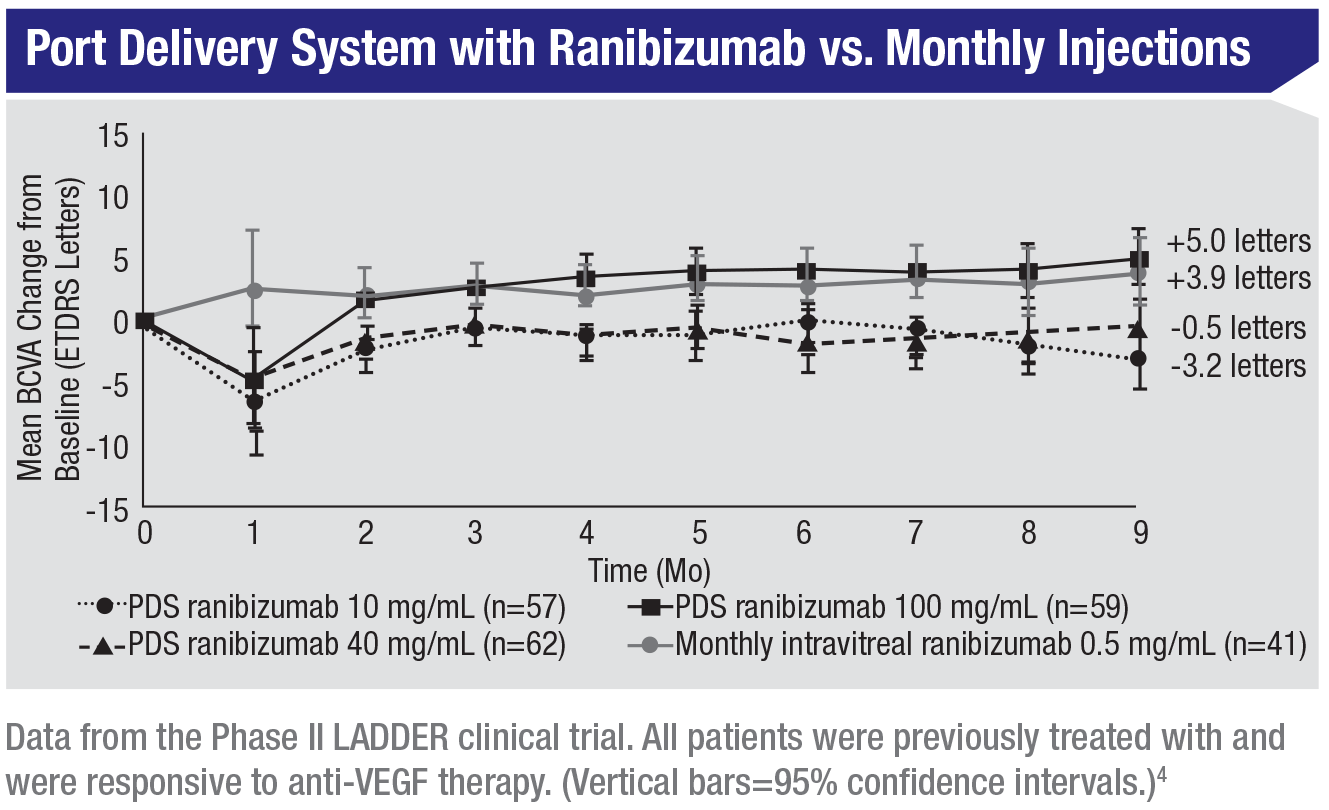
Dr. Rosenfeld says that data from the LADDER study, involving Genentech’s Port Delivery System with ranibizumab, was presented at the FLORetina meeting. “It appears to provide some extended benefit for patients,” he says. “Less-frequent dosing was needed. In addition, there’s a sustained-release tyrosine kinase inhibitor from Graybug Vision (Redwood City, California) that may or may not prove to be a long-term solution for this problem.”
• Home monitoring. “The key to monitoring those lesions is going to be some kind of home monitoring that’s reliable,” notes Dr. Rosenfeld. “Our dream device would be an OCT that a patient could use every day, or every other day. That would allow us to identify exudation as soon as it develops. Home OCT monitoring would be useful for following patients who have non-exudative, neovascular lesions that we can identify with OCT angiography, who are at high risk for exudation, as well as patients on a treat-and-extend regimen. If they were testing themselves with home OCT monitoring, we’d know as soon as the fluid develops or recurs.
“Notal Vision has a prototype that’s being tested that uses an AI algorithm capable of picking up exudation,” he continues. “There are other strategies being developed as well, but the Notal Vision device may become available within a year or two. Patients wouldn’t buy the instrument; it would be more of a lease situation. Medicare currently covers the cost of home monitoring, so for a nominal fee, perhaps in addition to Medicare coverage, you’d be able to have your patients monitored at home.”
• Gene therapy. “Two companies have gene therapies that involve injecting a viral vector into the eye,” says Dr. Freund. “Adverum Biotechnologies is in a Phase I trial with an intravitreal injection, and Regenxbio will soon be conducting a Phase II trial involving subretinal delivery of gene therapy. The viral vector inserts DNA into cells in the eye, so they start to produce either ranibizumab or aflibercept on their own. Theoretically, you could inject it once and you’d be done. To me that’s very exciting.” REVIEW
Dr. Freund is a consultant for Allergan, Novartis, Zeiss, OptoVue and Heidelberg Engineering, and receives research support from Genentech Roche. Dr. Rosenfeld has received research funding from and is a consultant for Carl Zeiss Meditec.
1. Emerson GG. Silicone oil droplets are more common in fluid from BD insulin syringes as compared to other syringes. Journal Vitreoretinal Diseases 2017;1:6:401-406.
2. Sharma A, Kumar N, Bandello F, Loewenstein A, Freund KB. Understanding intravitreal silicone oil droplets due to intravitreal injections. Retina 2019;39:7:1233-1235.
3. Singh RP, Stone TW, eds. 2018 Global Trends in Retina Survey: Chicago, IL. American Society of Retina Specialists; 2018.
4. Campochiaro PA, Marcus DM, Awh CC, et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration: Results from the randomized Phase 2 Ladder clinical trial. Ophthalmology 2019 Apr 1. pii: S0161-6420(18)33328-1. doi: 10.1016/j.ophtha.2019.03.036. [Epub ahead of print]



