The past 15 years have seen a lot of change in corneal transplantation for conditions such as Fuchs’ endothelial dystrophy, with endothelial transplant methods taking over from penetrating keratoplasty. However, there’s an even newer wave of transplant techniques on the horizon, which may help decrease the amount of tissue needed to treat patients while maintaining safety and efficacy. Here, we’ll detail the latest techniques surgeons are working on to circumvent this problem, as well as to help decrease tissue rejection.
The Push for New Techniques
Also in this Issue... Artificial Intelligence for the Cornea Specialist by Christine Yue Leonard Premium IOLs in Patients with Corneal Conditions by Asim Piracha, MD Diagnosis & Management of Blepharitis by Charles Bouchard, MD, MA |
Human corneal transplantation has substantially evolved due to the improvement of surgical technique since its first successful attempt in 1905. The paradigm of the surgical approach has shifted from full-thickness transplant to partial-thickness transplantation for specific diseased tissue layers. Over the past few decades, endothelial keratoplasties have begun to supplant penetrating keratoplasty as the most common corneal transplant procedure. Descemet’s membrane endothelial keratoplasty is a particularly common EK procedure that involves selective transplantation of only the endothelium and Descemet’s membrane without stroma. The transplantation of less tissue in DMEK allows for minimal surgical manipulation, more predictable outcomes and enables faster restoration of the corneal anatomy with fewer complications. However as one may predict, the increasing success of EKs comes with an increasing demand for donor corneas, for which there is currently a severe global shortage.
Descemet’s Stripping Only
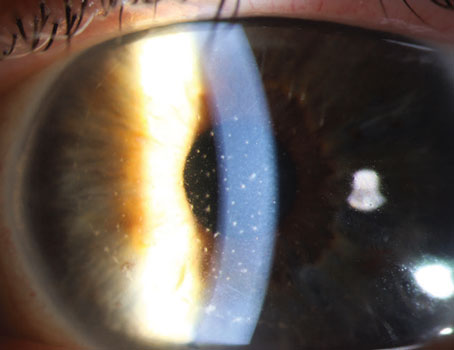
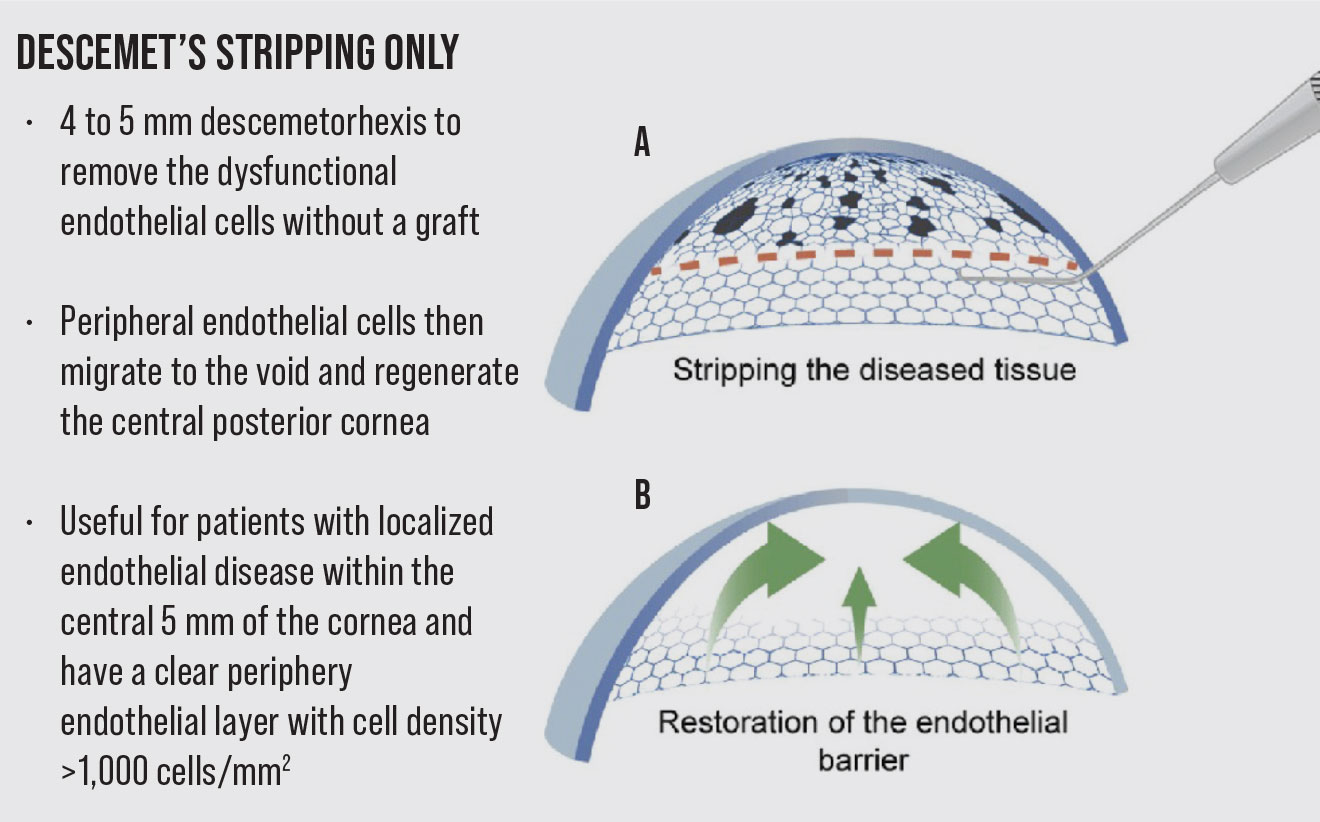
To circumvent the need for donor tissue and risk of possible graft rejection, the procedure of Descemet Stripping Only was introduced. It involves a descemetorhexis to remove the dysfunctional endothelial cells without a graft. The peripheral endothelial cells then migrate to the stripped area and regenerate the central posterior cornea.1,2 The DSO procedure is considered a viable treatment option for patients who have localized endothelial disease within the central 5 mm of the cornea and have a clear periphery endothelial layer with cell density >1,000 cells/mm2. Compared to an 8-mm descemetorhexis traditionally performed in DMEK, the descemetorhexis in DSO is usually only 4 to 5 mm. Another benefit of this procedure is that there’s no need to use long-term topical corticosteroids to prevent graft rejection, and hence there is no potential risk of steroid-induced intraocular pressure elevation.
 |
 | ||
|
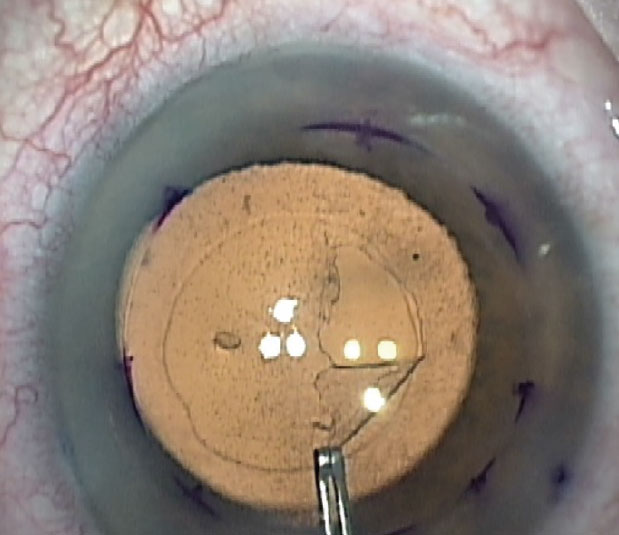
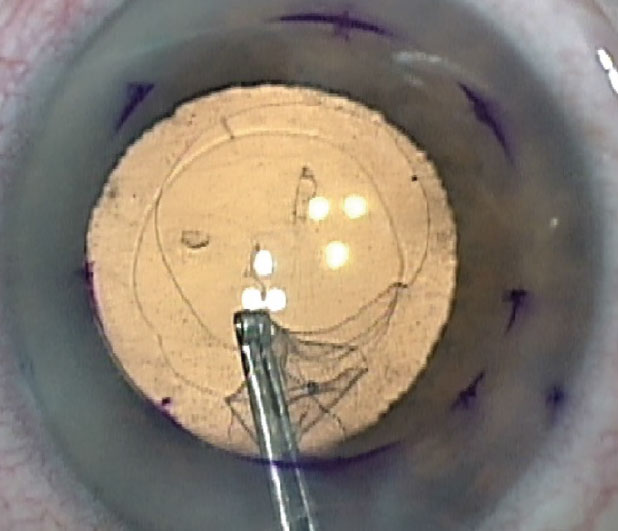
The initial part of the Descemet’s Stripping Only procedure. Also seen is the pre-existing anterior capsulorhexis opening. |
|
The feasibility of this procedure is based on prior ex vivo studies and clinical observations.1-3,5-10 Endothelial cells derived from Fuchs’ endothelial cell disease patients have been shown to proliferate in vitro without viral transduction.3 The cells are generally thought to proliferate until they come into contact with one another, e.g., contact inhibition, at which point they go into cell cycle arrest. When comparing endothelial cells isolated from the central cornea vs peripheral cornea, peripheral cells have a higher proliferation rate when grown ex vivo or in vitro. Hence, these peripheral cells are thought to migrate to the central cornea after DSO and then proliferate in the space. Clinically, this has been observed by one group of researchers, who reported migration of endothelial cells into denuded corneal stroma after DMEK in five patients with Fuchs’ endothelial dystrophy.1
A recent review has summarized the outcomes of 47 eyes with Fuchs’ endothelial cell disease that underwent DSO.4 The patient population had an average age range from 34 to 91 years, and the diameter of the descemetorhexis ranged from 4 to 9 mm. Postop, a clear cornea was observed in 65 percent of eyes, with improvement noted within a month. However, the success rate has been variable. One study reported a corneal clearing with improved visual acuity in three of eight cases that underwent DSO with cataract surgery,5 although all patients eventually underwent EK.
 |
On the other hand, another study reported 10 of 13 eyes with resolution of corneal edema with an intact central endothelial mosaic at six months and only three eyes had to eventually undergo EK.6 Similarly, in nine patients who underwent DSO with or without cataract surgery, the average time to achieve recovery of vision was 6.5 weeks.7 However these patients also had a 10-percent decrease in peripheral endothelial cell count over the 12-month postop period. A separate group performed a retrospective study to determine predictive factors that may lead to better outcomes after DSO.8 However, their review failed to show any significant predictive factors between success and failure groups based on age, pachymetry and endothelial cell count. Nonetheless, many authors have suggested there are better outcomes in general when a smaller central descemetorhexis (4 mm) is performed.
Overall, DSO is a relatively new treatment option with early data suggesting that it’s effective in Fuchs’ patients with localized endothelial disease within the central 5 mm of the cornea. While DMEK or DSEK remains the gold standard, DSO provides an alternative if donor tissue isn’t readily available, graft rejection is a concern or postoperative steroid use is undesirable. Surgeon experience and focus are important when performing the gentle descemetorhexis necessary to reduce any roughening of the posterior stroma that could impede endothelial cell migration and repopulation.
Cultured Endothelial Cell Transplants
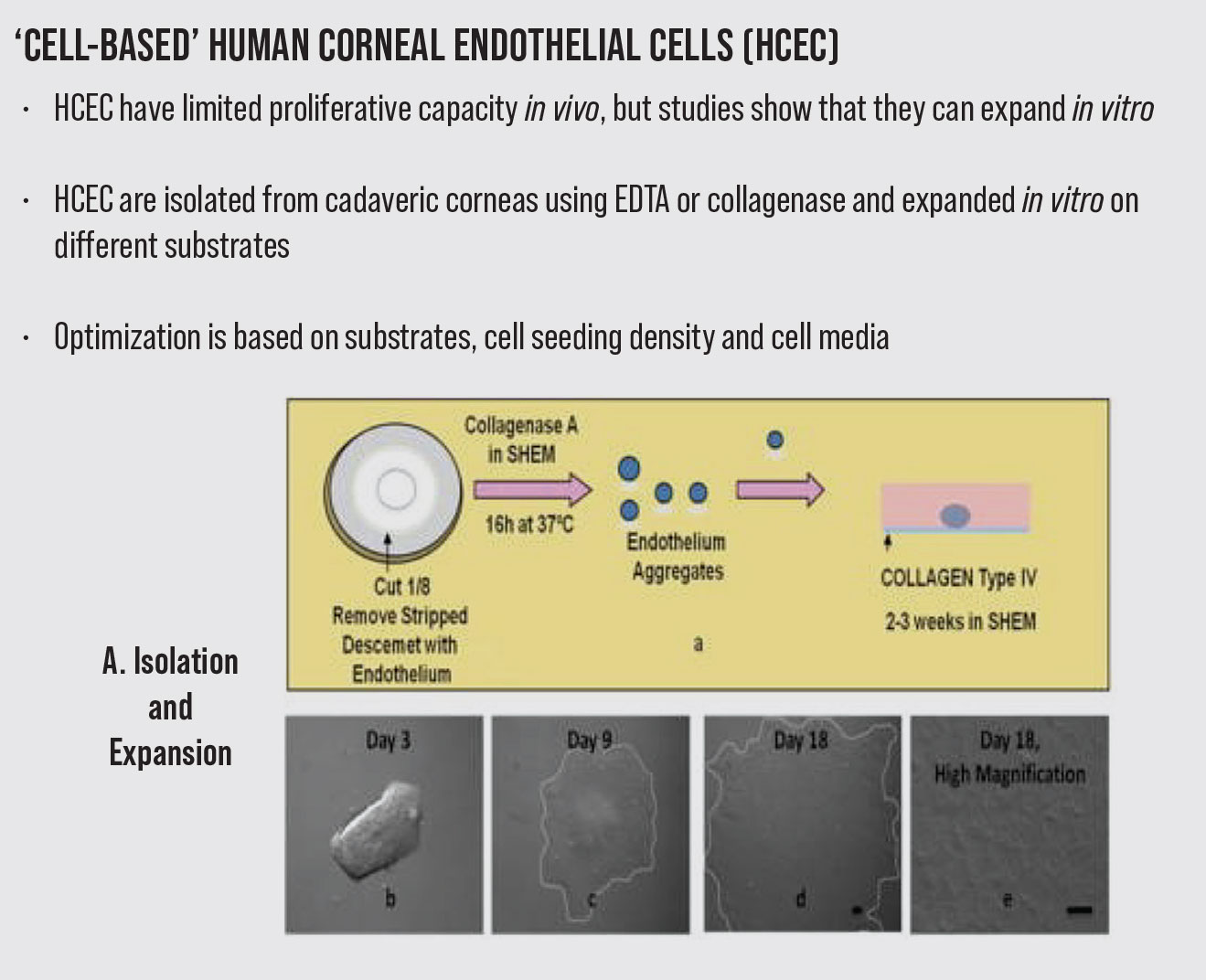
Another new treatment option as an alternative to replacing damaged endothelial cells is the transplantation of engineered corneal tissue created through endothelial cells expansion.11 This novel approach relies on the in vitro proliferation of isolated corneal endothelium cells (CECs), which is an attractive way of providing an unlimited source of cells to overcome the donor cornea shortage. However, the engineering technology used to promote endothelial cell proliferation in vitro and facilitate in vivo transplantation is challenging. Although human corneal endothelial cells have limited proliferative capacity as the cell cycle held in the G1 phase in vivo, studies showed that they can expand in vitro.12-14 Understanding endothelial cell biology and using promoting factors can thus provide an effective way to master the engineering technique.
Corneal endothelium engineering procedures include crucial steps such as tissue removal, cell isolation, culture and implantation to the recipient’s eyes with or without a carrier. Different sources of autologous or heterologous cells have been used in corneal endothelium expansion.14-20 The crucial requirement for endothelial cell culture is a source of viable, proliferative cells. Primary corneal endothelial cells isolated from cadaver donors, peripheral corneal endothelial stem cells or organ-specific adult stem cells can be used as good sources for corneal endothelial cell culture.
The Importance of the Endothelium Corneal endothelial cells are embryonically derived from cranial neural crest cells and form a single monolayer of hexagonal cells lining Descemet’s membrane. These cells play a pivotal role in regulating corneal stromal hydration and hence transparency by exerting effective barrier and pump functions. Unlike endothelial cells from other species, human endothelial cells have limited proliferative capacity in vivo. Hence, the loss of cells or cell dysfunction has a dramatic impact on the overall transparency of the cornea as newer cells are hard to replicate and maintain the pump and barrier function. When the cell density decreases to <500 cells/mm2, the endothelial cell pump function underperforms and the cornea becomes edematous. The current treatment for corneal blindness caused by dysfunctional endothelial cells usually resorts to transplantation, wherein new endothelial cells are brought into replace the lost or dysfunctional cells. |
Cadaver donor characteristics such as age, proliferative capacity and cell density significantly determine the success of cell culture. Of note, in one study, young donors showed higher endothelial cell density, better proliferative capability and cell homogeneity than old-aged donor corneas.14 The study categorized young donor corneas as being from donors under age 30 and the old-aged cornea as those from donors older than 50. The endothelial cell density at age 20 to 40 is about 3,000 cells/mm2, and decreases to around 2,700 cells/mm2 at 50 to 70 years of age. In addition, young donors have more proliferative cells (47 percent) than the older donor group (23 percent). While human corneal endothelial cells from young donors expressed “Ki-67” 36 hours after wounding, indicating that cells were active in the late G1 phase through to the M phase to represent cell proliferation, the endothelial cells from older donors delayed cell proliferation until 48 hours. A study revealed that endothelial cells from donors could be expanded up to the third passage while maintaining their polygonal cell shape.21-23 However, cultured human corneal endothelial cells from old donors lost their unique polygonal morphology and presented more heterogeneity in the fourth and fifth passages when compared to young donors. Although corneal endothelial cells from young donors are preferred to optimize in vitro cell culture, most young donor corneas won’t be easy to obtain because they are consumed in clinical corneal transplantation. Thus, in vitro culturing cells from old-aged donors becomes relatively challenging.24
The cells from the peripheral endothelium (2.75 mm) can be an alternative resource other than the central cornea (8.25 mm). Peripheral corneal endothelial cells with the proliferative potential to differentiate into mature endothelial cells have been referred to as human corneal endothelial progenitor cells. These progenitor cells, expressed neural crest cell markers, p75 Neurotrophin receptor, SOX9, FOXC2, stem/progenitor markers (Sox2, Lgr5, CD34, Pitx2, and telomerase), are found in the peripheral and transition zones of the cornea. Other specific stem cell markers (Nestin, ALP, and telomerase) are found at the trabecular meshwork and the transitional zone between trabecular meshwork and the peripheral area of the cornea.25 After wounding, peripheral corneal endothelial cells presented proliferative marker expression (BrdU and TGF-β), and increased differentiation markers (Pax6, and Sox2), whereas Oct-3/4 was found in the trabecular meshwork and Wnt-1 in both trabecular meshwork and transitional zones.
As immune-mediated graft rejection can be avoided by providing cultivated autologous corneal endothelium cells, we focus on autologous, easily accessible adult stem cells. Studies reported that organ-specific adult stem cells used for endothelial cell culture are from adipose tissue, umbilical cord blood or bone marrow, directed differentiation competent embryonic stem (ES) cells, induced pluripotent stem cells (iPSC), skin-derived precursors (SKP) and mesenchymal stem cells (MSCs).14-20 However, the concerns with culturing these stem cells are that both mechanisms of corneal endothelial cell embryonic developmental steps and cell reprogramming-differentiation pathways remain unclear. They may present heterogeneous proliferative and differentiation capacities and unexpected morphological results after differentiation, thus hindering the development of the various culturing protocols.26
Currently, the most established protocol for cell isolation consists of peel-and-digest method.27 In this method, the Descemet’s membrane endothelial donor is peeled from the stroma, followed by dissociation of cell junctions to separate the corneal endothelial cells from the membrane.
Enzymatic or nonenzymatic tissue digestion strategies are employed. The tissues are treated with collagenase, trypsin or dispase in the enzymatic digestion procedures, while the nonenzymatic digestion uses ethylenediaminetetraacetic acid (EDTA) to break cell–cell junctions. The main composition of extracellular matrix (ECM) collagens, which help human CECs adhere to DM, is type IV collagen. Therefore, the most promising enzyme for isolating endothelial cells is collagenase. More viable cells are isolated after collagenase digestion than after trypsin digestion. Collagenase induces a selective reduction of the intercellular matrix with minimal damage to cell membranes, whereas trypsin mainly acts on the intracellular mucoproteins, thus affecting the cell membrane. The mechanism of action of EDTA is to release cell junctions but it also enhances cell division upon exposure to mitogens. Not surprisingly, EDTA is often used with trypsin to disrupt cell contacts to separate cell-to-cell contact and to separate cells from the ECM without undesirable effects on cell viability.
The isolated cells are then collected and plated onto coated plates in the medium that enhances attachment. Studies reported optimal seeding plate densities ranging widely from 10,000 to 25,000 cells/cm2 to maintain morphology but obtain a sufficient number of cells in the endothelial cells’ passages.
In corneal tissues, endothelial cells adhere to DM via ECM components. ECM comprises a range of structural proteins (collagen I, elastin), adhesive proteins (collagen IV, VIII, fibronectin, laminin) and glycosaminoglycans (GAG). They construct a scaffolding to provide mechanical support for cell adhesion and organization. To mimic a physiological environment for endothelial cell plating and growth, the cell adhesion coating derived from these ECM proteins is applied on the surface of the tissue culture substrate. Tissue culture plates are coated with different combinations of ECM proteins and compared to uncoated plates to assess the effects on the cultivated endothelial cells.28-33 However, due to different techniques and applied cell culture protocols, some studies showed conflicting results.
Although there is no consensus on the optimal coating, all proteins (fibronectin, poly-D-lysine, collagen type I, fibronectin/collagen I, collagen IV, and FNC coating mix, laminin) demonstrated increased endothelial cell adhesion compared to uncoated controls. Fibronectin-coated culture plates have been shown to improve cell spreading, reduce cell loss after rinsing, and promote better cell confluence and morphology compared to cultures grown on fibronectin-coated plates, collagen IV-coated plates and uncoated plates. Thus, currently the most used coating is the fibronectin coating mix (a mixture of fibronectin, collagen, and albumin).
Synthetic and semi-synthetic materials are being developed to provide a stable, well-defined, easily usable polymer scaffold to facilitate initial cell adhesion and detachment of cultivated cells.30, 33 However, the drawbacks of synthetic and semi-synthetic materials include fragility, requiring another biomaterial for transplant; unpredictable results on cells affected by temperature change; and difficulty in scaling them up.
 |
Many endothelial cell culture media combinations, including base media, essential and nonessential amino acids, growth factors and vitamins, have been described.34-37 However, no single medium is superior. A study introduced dual media that uses both maintenance and proliferation media. Maintenance media consist of basal media but without growth factors, whereas proliferation media is basal media with growth factors. After cell isolation, endothelial cells are cultured in maintenance media overnight, followed by proliferation media to promote cell growth. Maintenance media has replaced proliferation media for stabilizing the cells after the cells reach confluence. Studies have shown that the endothelial characteristics were maintained in the dual but not the single media.36 For cultured endothelial cells to be applied in clinical transplantation, preventing endothelial-to-mesenchymal transition (EMT) is crucial. EMT leads to irreversible transdifferentiation from an endothelial to a mesenchymal phenotype, resulting in a fibroblastic phenotype and a loss of cell-cell contacts and hexagonal morphology. One study showed that EMT is induced by TGF-β, FGF-2 and IL-1β.
During EMT, the presence of α-smooth muscle actin (α-SMA) promoted by TGF-β indicates reorganization of the cytoskeleton and fibrosis.TGF-β arrests the cell cycle at the G1 stage, inhibiting proliferation and inducing differentiation. Hence, anti-TGF-β inhibitor SB-431542 avoids EMT by blocking the TGF-β receptor in cultivated endothelial cells to allow cell expansion.38 Adding an anti-TGF-β inhibitor to the culture media can promote cell proliferation and prevent EMT. In addition, the antioxidation derivative of ascorbic acid, L-ascorbic acid 2-phosphate (A-2P), can stimulate the barrier function in endothelial cell monolayers and enhance various cell growth effectively. Fibroblast growth factor-2 (FGF-2) is a well-known growth factor used to supplement the culture media of many cell types. However, FGF-2 may induce EMT despite some studies showing that combining A-2P and FGF-2 significantly increased the growth of cultivated endothelial cells. Other studies have shown success of culturing CEC monolayers derived from collagenase digestion of stripped Descemet’s membrane in modified embryonic stem cell medium supplemented with p120 and Kaiso siRNAs.40-45 This reprogramming approach circumvents the need of using iPSCs or embryonic stem cells. Overall, there are many combinations of cell culture techniques and there continues to be development of new techniques to avoid EMT and promote cultivated cell growth.
After the successful cell culture, the cultivated corneal endothelial cells can be directly injected into the eye. Alternatively, the formed cell sheet from cultivated cells is transferred to substrates or carriers that provide mechanical support for the transplantation. For the direct cell injection method, the patient must remain in a prone position for hours to allow cell adherence to the posterior cornea.
To promote endothelial cell adhesion, researchers have injected a Rho-kinase (ROCK) inhibitor along with the cultivated cells.39 (The RAS homologous [Rho] protein family is a member of the RAS superfamily of small guanosine triphosphatases [GTPases]). Animal studies proposed that Y-27632, a selective ROCK inhibitor, promoted cell proliferation and inhibited cell apoptosis in corneal endothelial cells. A recent clinical study using ROCK inhibitor-supplemented cell suspension demonstrated endothelial cell density increment and formation of a monolayer sheet of the endothelium. Despite these encouraging results, this direct cell injection method may be limited by clinical feasibility and raises concern about the systemic dissemination risk. The carrier implantation method, on the other hand, is still in the preclinical stage.
An ideal carrier would facilitate cultivated endothelial cells’ adhesion to the posterior stroma and mimic the characteristics of the DM to support the interaction between the endothelial cells and the recipient stroma. Different carriers have been extensively investigated. They include:
- biologically derived (such as amniotic membrane, silk fibroin, human anterior lens capsules, decellularized DM or stroma, fish scales or plastic compressed collagen/gelatin);
- synthetic (such as chitosan blends, Poly-ε-lysine peptide, Polymethylmethacrylate hydrogel, Polylactic-co-glycolic acid, Polycaprolactone); and
- semisynthetic (Gelatin methacrylate, chitosan).
Despite carrier materials advancement, cellular properties remain a limiting factor in terms of transparency, permeability, sufficient mechanical strength, flexibility to adjust to the corneal curvature and biocompatibility.40 The carrier with cultivated endothelial cells is implanted into the anterior chamber using a DMEK surgical technique. The placement of the fragile monolayer endothelial cell sheet in the anterior chamber and firmly fixing it to the posterior cornea is one of the main surgical challenges.
After attachment of cultivated endothelial cells to the recipient’s posterior stroma, the carrier needs to enhance endothelial cells’ DM production to ensure stromal transparency after transplantation. Further in vivo and animal studies can investigate surgical transplantation or implantation of cultivated endothelial cells with or without carriers, postsurgical DM production, immune tolerance and long-term outcomes.
In conclusion, new advancements targeting endothelial donor tissue shortage with DSO and cultivated endothelial cells are emerging. This review highlights in vitro expansion of corneal endothelial cells isolated from cadaver donors, peripheral corneal endothelial cells or organ-specific adult stem cells. Collagenase digestion is an effective way to isolate the corneal endothelial cells. Cell plating density can be different between studies.
Combined culture media have also been described, including dual media or use of siRNAs with various growth factors attempted to promote cell growth and inhibit EMT. However, it remains challenging to culture corneal endothelial cells on the coating surface while maintaining their characteristics. Although several types of carriers and surgical techniques have been proposed for cultivated EC transplantation, further studies need to develop ways to resolve biocompatibility issues.
Dr. John is in private practice in the Chicago area, and is a clinical associate professor, Loyola University at Chicago. Dr. Cheng is a resident at Broward Health, Department of Ophthalmology, Fort Lauderdale, and a voluntary clinical associate professor at Florida International University, Department of Ophthalmology in Miami, and a clinical assistant professor at Florida International University.
The authors would like to thank Sean Tighe for his assistance in preparing this paper.
The authors have no financial interest in any of the products mentioned.
1. Jacobi C, Zhivov A, Korbmacher J, Falke K, Guthoff R, Schlötzer-Schrehardt U, Cursiefen C, Kruse FE. Evidence of endothelial cell migration after Descemet membrane endothelial keratoplasty. Am J Ophthalmol 2011;152:4:537-542.
2. Arbelaez JG, et al. Long-term follow-up and complications of stripping Descemet membrane without placement of graft in eyes with Fuchs endothelial dystrophy. Cornea 2014;33:1295-1299.
3. Zaniolo K, Bostan C, Rochette Drouin O, et al. Culture of human corneal endothelial cells isolated from corneas with Fuchs endothelial corneal dystrophy. Exp Eye Res 2012;94:1:22-31.
4. Van den Bogerd B, Dhubhghaill SN, Koppen C, et al. A review of the evidence for in vivo corneal endothelial regeneration. Surv Ophthalmol 2018;63:2:149-165.
5. Bleyen I, et al. Spontaneous corneal clearing after Descemet’s stripping. Ophthalmology 2013;120:1:215.
6. Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea 2016;35:10:1267–73.
7. Macsai MS, Shiloach M. Use of topical rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea 2019;38;5:529-534.
8. Davies E, Jurkunas U, Pineda R. Predictive factors for corneal clearance after descemetorhexis without endothelial keratoplasty. Cornea 2018;37;2:137-140.
9. Koenig SB. Planned descemetorhexis without endothelial keratoplasty in eyes with Fuchs corneal endothelial dystrophy. Cornea 2015;34:1149-51.
10. Moloney G, et al. Descemetorhexis without grafting for fuchs endothelial dystrophy- supplementation with topical ripasudil. Cornea 2017;36:642-648.
11. Okumura N, Kinoshita S, Koizumi N. Cell-based approach for treatment of corneal endothelial dysfunction. Cornea 2014;33:11:S37-41.
12. Spinozzi D, Miron A, Bruinsma M, et al. New developments in corneal endothelial cell replacement. Acta Ophthalmol 2021;99:7:712-729.
13. Zavala J, López Jaime GR, et al. Corneal endothelium: Developmental strategies for regeneration. Eye (Lond) 2013;27:5:579-88.
14. Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci 2000;41:3:660-7.
15. Gao Y, Zhou Q, Qu M, et al. In vitro culture of human fetal corneal endothelial cells. Graefes Arch Clin Exp Ophthalmol 2011;249:5:663-9.
16. Shao C, Fu Y, Lu W, Fan X. Bone marrow-derived endothelial progenitor cells: a promising therapeutic alternative for corneal endothelial dysfunction. Cells Tissues Organs 2011;193;4:253-63.
17. Okumura N, Kusakabe A, Hirano H, et al. Density-gradient centrifugation enables the purification of cultured corneal endothelial cells for cell therapy by eliminating senescent cells. Sci Rep 2015;7:5:15005.
18. Parekh M, Ahmad S, Ruzza A, Ferrari S. Human corneal endothelial cell cultivation from old donor corneas with forced attachment. Sci Rep 2017;7:1:142.
19. Parekh M, Romano V, Ruzza A, et al. Increasing donor endothelial cell pool by culturing cells from discarded pieces of human donor corneas for regenerative treatments. J Ophthalmol Jul 21;2019:2525384. doi: 10.1155/2019/2525384. eCollection 2019.
20. Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev 2014;23:12:1340-54.
21. Miyata K, Drake J, Osakabe Y, et al. Effect of donor age on morphologic variation of cultured human corneal endothelial cells. Cornea 2001;20:1:59-63.
22. Chen KH, Azar D, Joyce NC. Transplantation of adult human corneal endothelium ex vivo: A morphologic study. Cornea 2001;20:7:731-7.
23. Roy O, Leclerc VB, Bourget JM, Thériault M, Proulx S. Understanding the process of corneal endothelial morphological change in vitro. Invest Ophthalmol Vis Sci 2015;19:56:2:1228- 37.
24. McGlumphy EJ, Margo JA, Haidara M, et al. Predictive value of corneal donor demographics on endothelial cell density. Cornea 2018;37:9:1159-1162.
25. Okumura N, Hirano H, Numata R, et al. Cell surface markers of functional phenotypic corneal endothelial cells. Invest Ophthalmol Vis Sci 2014;55:11:7610-8.
26. Wongvisavavit R, Parekh M, Ahmad S, Daniels JT. Challenges in corneal endothelial cell culture. Regen Med 2021;16:9:871-891.
27. Faye PA, Poumeaud F, Chazelas P, et al. Focus on cell therapy to treat corneal endothelial diseases. Exp Eye Res 2021;204:108462.
28. Ishino Y, Sano Y, Nakamura T, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Invest Ophthalmol Vis Sci 2004;45:3:800-6.
29. Kabosova A, Azar DT, Bannikov GA, et al. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 2007;48:11:4989-99.
30. Shah A, Brugnano J, Sun S, Vase A, Orwin E, 2008. The development of a tissue engineered cornea: Biomaterials and culture methods. Pediatr Res 2008;63:5:535-44.
31. Suda T, Nishida T, Ohashi Y, et al. Fibronectin appears at the site of corneal stromal wound in rabbits. Curr Eye Res 1981;1:9:553-6.
32. Parekh M, Romano V, Hassanin K, et al. Biomaterials for corneal endothelial cell culture and tissue engineering. J Tissue Eng 2021;12:2041731421990536.
33. Navaratnam J, Utheim TP, Rajasekhar VK, Shahdadfar A. Substrates for expansion of corneal endothelial cells towards bioengineering of human corneal endothelium. J Funct Biomater 2015;6:3:917-45.
34. Lu X, Chen D, Liu Z, et al. Enhanced survival in vitro of human corneal endothelial cells using mouse embryonic stem cell conditioned medium. Mol Vis 2010;16:611-22.
35. Chen P, Chen JZ, Shao CY, et al. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp Ther Med 2015;9:2:351-360.
36. Peh GS, Chng Z, Ang HP, et al. Propagation of human corneal endothelial cells: a novel dual media approach. Cell Transplant 2015;24:2:287-304.
37. Mannagh J, Irving AR. Human corneal endothelium: Growth in tissue cultures. Arch Ophthalmol 1965;74:6:847-9.
38. Okumura N, Kay EP, Nakahara M, et al. Inhibition of TGF-B signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One. 2013;8:2:e58000. doi: 10.1371/journal.pone.0058000. Epub 2013 Feb 25.
39. Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci 2009;50:8:3680-7.
40. Tsai MC, Daniels JT. The impact of biomechanics on corneal endothelium tissue engineering. Exp Eye Res 2021;209:108690.
41. Zhu Q, Zhu Y, Tighe S, et al. Engineering of human corneal endothelial cells in vitro. Int J Med Sci 2019;16:4:507-512.
42. Chen S, Zhu Q, Sun H, et al. Advances in culture, expansion and mechanistic studies of corneal endothelial cells: A systematic review. J Biomed Sci 2019;26:1:2.
43. Lu WJ, Tseng SC, Chen S, et al. Senescence mediated by p16INK4a impedes reprogramming of human corneal endothelial cells into neural crest progenitors. Sci Rep 2016;14;6:35166.
44. Li Z, Tighe H, Chen Z, Tseng L. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J Cell Biol 2014; 206:6:799–811.
45. Zhu YT, Li F, Han B, et al. Activation of RhoA-ROCK-BMP signaling reprograms adult human corneal endothelial cells. J Cell Biol 2014;206:6:799- 811.
 |