As small-gauge vitrectomy systems and instrumentation have improved dramatically over the last decade, vitreoretinal surgeons have been called upon to intervene earlier for the surgical treatment of epiretinal membrane. In this article we discuss the basics of ERM pathology, the surgical approaches vitreoretinal surgeons have in their arsenal, and when surgery may or may not be the right first choice for both the surgeon and the patient.
Pathophysiology
An epiretinal membrane is fibrocellular tissue found on the inner surface of the retina. Most ERMs are idiopathic. However, common identifiable causes include retinal vascular disease such as diabetic retinopathy, retinal vein occlusion, vitreous hemorrhage, ocular inflammation, trauma, prior intraocular surgery, retinal tear/detachment or retinal laser.1,2 The incidence of ERM varies widely in the literature, anywhere from 2.2 to 11 percent in phakic patients without pre-existing ocular conditions.3,4
In patients with idiopathic ERM, the proposed pathophysiology is that the vitreous liquefies and detaches from the retina—a posterior vitreous detachment—while the residual cortical vitreous serves as a scaffold for migration of microglial or retinal pigment epithelial cells onto the retinal surface. These cells later transdifferentiate into fibroblasts and form the ERM.5 In addition, an ERM may originate from residual cortical vitreous remnants that are sitting on the ILM surface which are activated into myo-fibroblasts and result in membrane formation.1
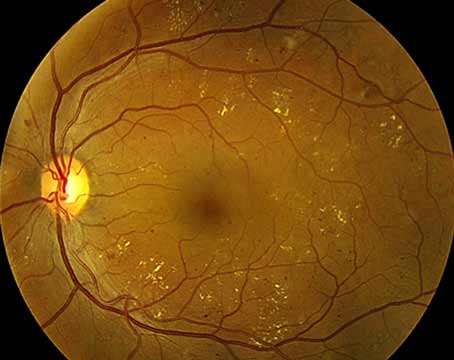
 |
| Figure 1. Loosely adherent ERM with many intervening spaces between the retina and epiretinal membrane. Preoperative OCT can be helpful in identifying these loose areas to help select the initial site of peel. |
Surgical Treatment of ERM
The mainstay of management of ERMs is surgical intervention with pars plana vitrectomy and ERM peeling (with or without concomitant internal limiting membrane peeling). All things considered, surgery is relatively safe, performed on an outpatient basis, and has a fast recovery time. Most patients who undergo surgery will have improved visual function postoperatively.6 However, most phakic patients will require cataract surgery within two years of vitrectomy. In many eyes, visual improvement is relatively slow—ranging from three to 12 months postoperatively. In addition, given the accelerated rate of nuclear sclerosis, the full potential of visual improvement isn’t seen until the patient becomes pseudophakic. Though manageable, the risk of peripheral retinal breaks isn’t negligible, occurring in 5 to 6 percent of cases.7 Some patients will achieve only partial improvement in vision or diminution of metamorphopsia, and a minority of patients may have diminished vision or contrast sensitivity postoperatively. Recurrence of ERMs may be seen in up to 5 percent of patients with idiopathic membranes. Risk factors for recurrence include young age, history of prior retinal detachment, and uveitis. In addition, lack of ILM peeling during surgery has been associated with increased risk of recurrence.
Comorbidities
In patients with diabetic retinopathy or retinal vein occlusion, the presence of an ERM can contribute to macular edema, confounding the decision-making for surgery. Most vitreoretinal surgeons would first initiate a series of intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections prior to considering surgical intervention to determine if there’s improvement in macular edema and/or visual function. If a patient’s visual symptoms persist, retinal traction remains on optical coherence tomography, or if there’s a taut posterior hyaloid, surgery may be considered. In addition, patients with pre-existing ocular inflammation must first have their inflammation treated adequately prior to considering ERM peel.
Considering Surgery
When considering surgical intervention, quantitative and qualitative measures can be used to help guide decision making. Visual acuity thresholds are only moderately helpful because metamorphopsia and disrupted binocular fusion are common symptoms not represented on the eye chart. The anatomic severity of ERM only loosely correlates to Snellen acuity. Generally speaking, patients with visual acuity of 20/20 or better can be observed. Moreover, patients who are asymptomatic or are mostly happy with their visual function can be monitored, regardless of baseline visual acuity. When patients start complaining of distortion, metamorphopsia, micropsia, poor binocular visual function or diplopia, and don’t have other identifiable causes for these complaints, surgery can be considered.
OCT is important for guiding decision-making. It allows for an objective assessment of the patient’s anatomy and for monitoring progression over time. Some prognostic markers have been found based on morphologic features on OCT. For example, UCLA’s Andrea Govetto and his co-workers demonstrated a reliable staging system for progression of ERM on OCT. Stage 1 ERMs were defined as thin and having a preserved foveal depression, whereas stage 4 ERMs were thick and associated with continuous ectopic inner foveal layers. As a patient progressed from stage 1 to stage 4, there was statistically significant diminished visual acuity.8 Other prognostic factors include the degree of surface wrinkling and tightly adherent membranes. Given these findings, the goal of surgery in some patients may be to halt further progression of the ERM, which can be thought of as equally as important as restoring lost vision.
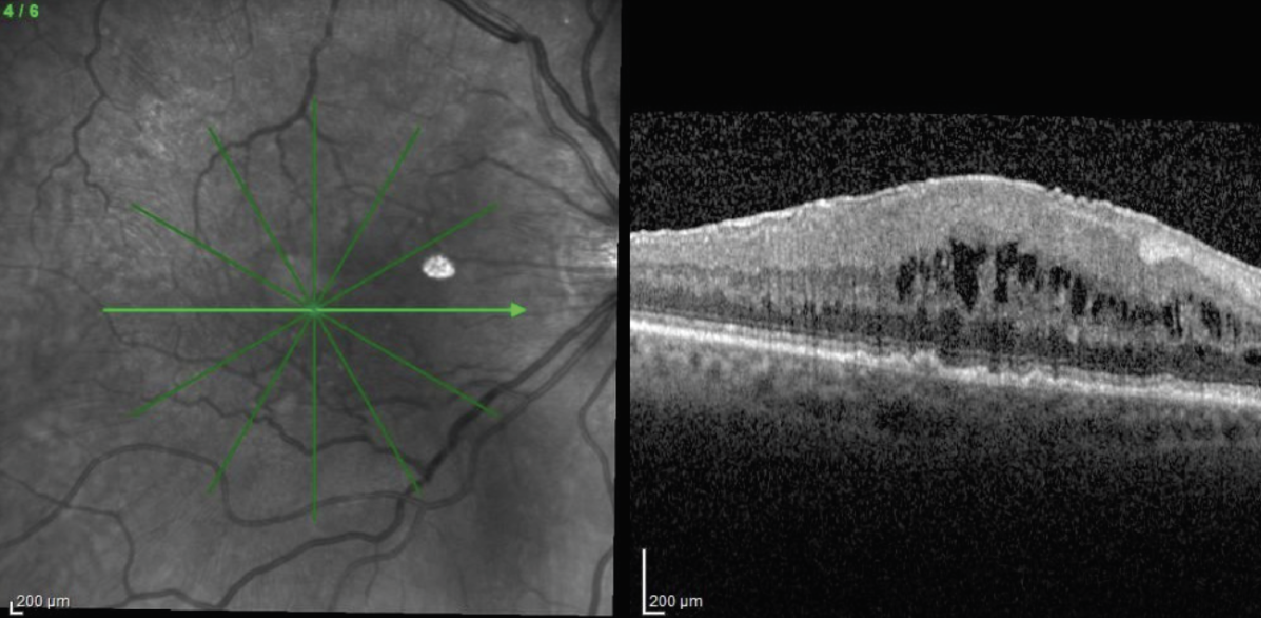 |
| Figure 2. Densely adherent ERM which is more tightly attached to the retinal surface and requires greater effort to peel. |
Surgical Technique
Much progress has been made in transconjunctival small-incision surgery and small-gauge instrumentation. Advances have helped aid in the safety and efficiency of surgery, but the traditional tenets of vitrectomy and membrane peeling remain the same. At the beginning of the case, core vitrectomy is performed and the posterior hyaloid is separated from optic nerve head and macula if they’re not already. At the end of the case, the peripheral retina must be thoroughly inspected and retinal breaks should be treated. Remember, retinal breaks underlie at least part of the pathophysiology of ERM, and many “idiopathic” ERMs probably arise due to existing peripheral breaks. Following are our tips for the surgery:
• Initiating the peel. Preoperative OCT can help guide where to initiate a peel, such as at elevations in the membrane. Some ERMs exhibit a loosely adherent morphology and are usually easily peeled, often being wholly removed in one (or a few) grabs (Figure 1). On the other hand, some membranes develop with broad and tight adherence to the retinal surface (Figure 2). Identifying these morphologies in advance can help tailor the choice for peeling instruments and reduce the surgeon’s frustration. When a membrane is tightly adherent, it can be tricky to initiate peeling, since it can require significant force to create the initial membrane fracture, as well as multiple re-grabs to propagate the peel. Because of their toughness, initiating the peel using a membrane scraper is difficult, so a forceps-first approach may be better.
When initiating a flap, one can align the forceps at the edge of the ERM and gently pinch, lift and release. Other options include initiating the peel with a Finesse flex loop (Alcon) or Tano diamond dusted membrane scraper (Synergetics/Bausch + Lomb). The peel can then be propagated using either forceps or a scraper.
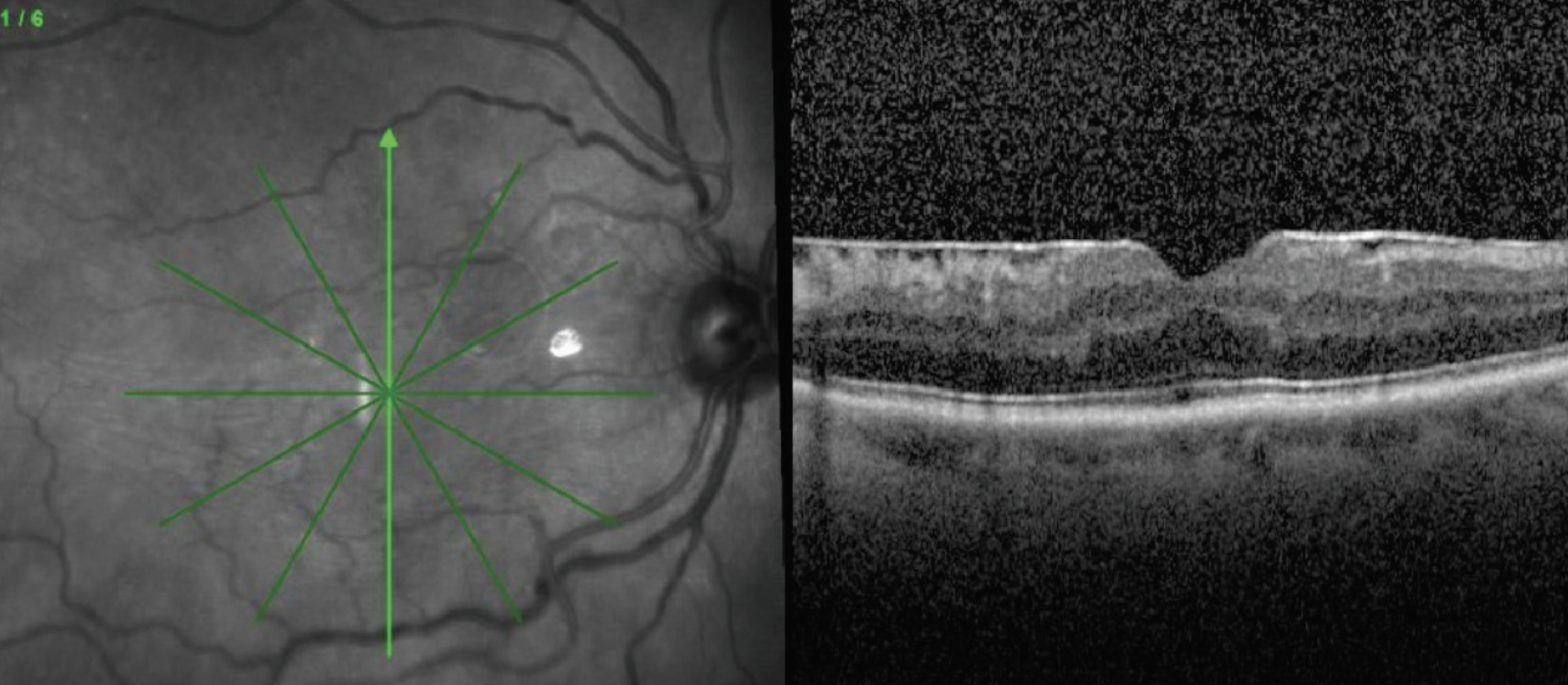
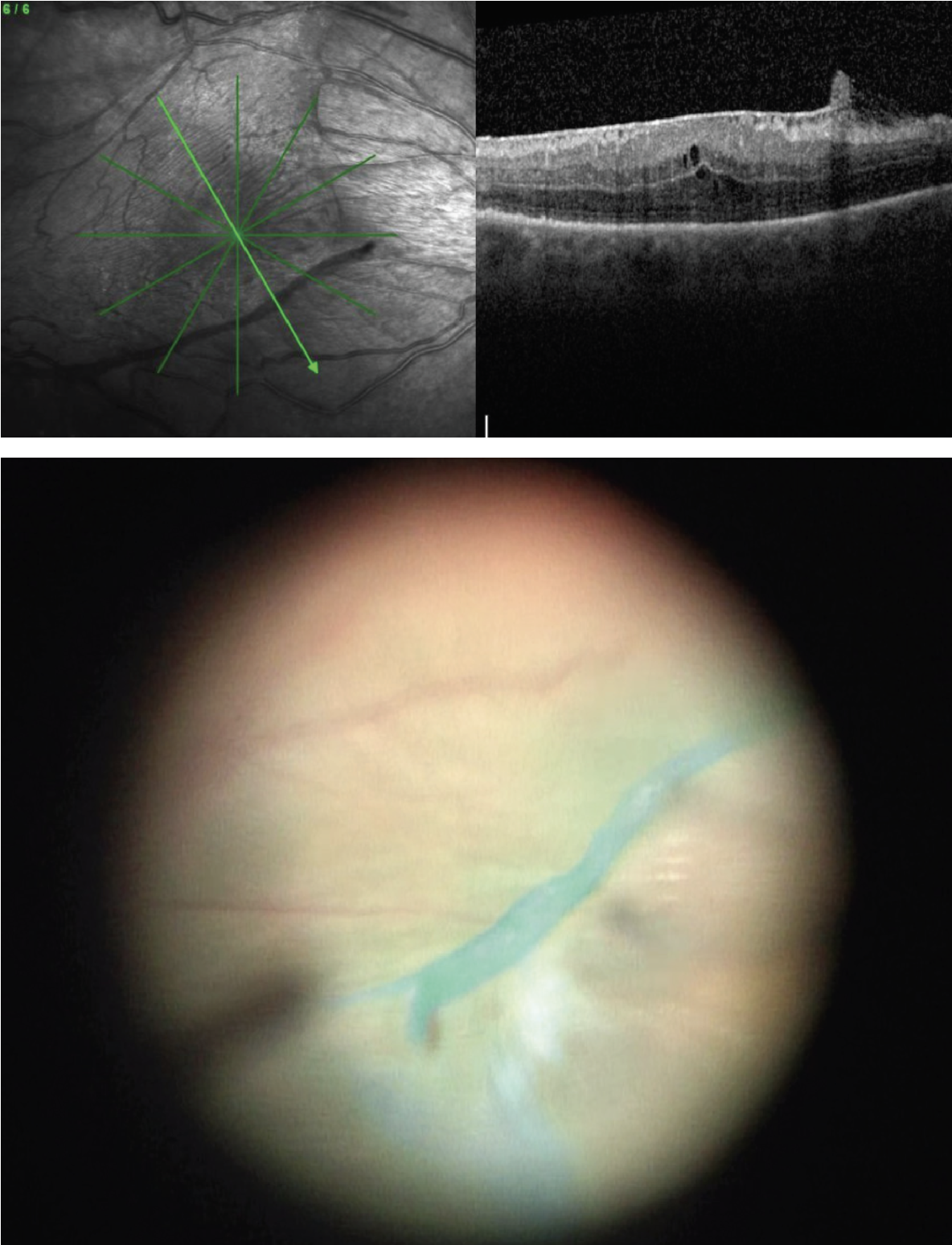 |
| Figure 3. Preoperative OCT and intraoperative view after staining with indocyanine green demonstrate a scrolled ILM edge which presents a natural and safe site for initiation of peel. |
• Visualization aids. Most surgeons also advocate for use of staining with triamcinolone or dyes such as indocyanine green or Brilliant Blue G (BBG). The microparticles of triamcinolone settle on the surface of the membrane, allowing you to visualize its edges and contour. Both ICG and BBG stain the ILM, allowing visualization of the interface between the ERM and ILM, acting as a “negative stain.” This can help the surgeon initiate the flap and help keep track of which areas have been peeled. Scrolled edges of ILM occur spontaneously due to ERM contraction and can be visualized either by OCT or intraoperatively as a prominent ridge of stained tissue (Figure 3). These areas are good targets for initiation of the peel since a large leaflet can be readily lifted, and the ridge allows for safe pinch-and-peel without contact of the retinal surface.
• To peel or not peel the ILM. Many surgeons routinely peel ILM along with ERM although others do not. The thought is that removal of the ILM releases traction to a greater extent and prevents recurrence. This is a topic of debate, however. A 2019 Preferred Practice Pattern published by the American Academy of Ophthalmology compared ERM peel alone to combined ERM and ILM peel. Five of the 10 studies reviewed demonstrated decreased risk of recurrent ERM in patients who underwent combined ERM/ILM peeling. Two studies didn’t find a difference in recurrence between the two methods of surgery.9 The prevention of recurrent ERM is probably the biggest justification for ILM peel, though other benefits may exist. ILM peeling may reduce the risk of recurrence of an ERM by ensuring all remnants are removed and by eliminating the scaffold on which ERMs form.10
Some evidence indicates that ILM peeling has better anatomic success compared to ERM peel alone, though there’s no difference in visual acuity outcomes between the two groups.11 Experience and preferred technique also guide surgeons when deciding on whether to peel the ILM. Some surgeons always peel it, others peel only the central parafoveal area, while others don’t routinely perform ILM peel. Other surgeons may employ ILM peel when other co-morbidities exist, such as diabetic macular edema.
In conclusion, whether to perform an ERM peel should be made on a case-by-case basis. Before peeling, ask yourself: Does the patient have co-morbidities such as DME or uveitis that can be first treated? If yes, then treat those first. If not, decide to what degree ERM impacts the patient’s quality of vision. Visual acuity is a helpful marker of overall visual function, but in general shouldn’t be used as cutoff for when surgery should be pursued. As noted, whether or not to peel the ILM is a topic of debate. Nonetheless, combined ILM/ERM peeling may be associated with a decreased risk of recurrence and better reconstitution of the foveal contour compared to ERM peel alone.
Dr. Regillo is the director of the Retina Service of Wills Eye Hospital, a professor of ophthalmology at Thomas Jefferson University School of Medicine and the principle investigator for numerous major international clinical trials.
Dr. Yonekawa is an assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University. He serves on the Education Committee of the American Society of Retina Specialists and on the Executive Committee for the Vit Buckle Society, where he is also the vice president for academic programming.
Dr. Pandit is a surgical retina fellow at Wills Eye Hospital.
Dr. Xu is an assistant professor of ophthalmology at the Sidney Kimmel Medical College at Thomas Jefferson University.
1. Bu SC, Kuijer R, Li XR, et al. Idiopathic epiretinal membrane. Retina 2014;34:12:2317-2335.
2. Cheung N, Tan SP, Lee SY, et al. Prevalence and risk factors for epiretinal membrane: The Singapore epidemiology of eye disease study. Br J Ophthalmol 2017;101:3:371-37.
3. Klein BR, Brown EN, Casden RS. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg 2016;42:4:537-541.
4. Zafar S, Siddiqui MAR, Shahzad R, et al. Swept-source optical coherence tomography to screen for macular pathology in eyes having routine cataract surgery. J Cataract Refract Surg 2017;3:3:324-327.
5. Foos RY. Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci 1977;16:5:416-22.
6. Ghazi-Nouri SM, Tranos PG, Rubin GS, Adams ZC, Charteris DG. Visual function and quality of life following vitrectomy and epiretinal membrane peel surgery. Br J Ophthalmol 2006;90:5:559-62.
7. Epiretinal Membrane: Outcomes. (n.d.). https://www.aao.org/focalpointssnippetdetail.aspx?id=af56d760-dd05-4399-ad95-b26636f5fc0c. Accessed December 31, 2022.
8. Govetto A, Lalane RA 3rd, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretinal membranes: Presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am J Ophthalmol 2017;175:99-113.
9. Idiopathic Epiretinal Membrane and Vitreomacular Traction Preferred Practice Patterns, American Academy of Ophthalmology; 2019;167-169.
10. Roh M, Kim EL, Eliott D, Yonekawa Y. Internal limiting membrane peeling during idiopathic epiretinal membrane removal: Pros and cons. Journal of VitreoRetinal Diseases 2017;1:2:138-143.
11. Fang X, Tong Y, Zhou Y, et al. Internal limiting membrane peeling or not: A systematic review and meta-analysis of idiopathic macular pucker surgery. British Journal of Ophthalmology 2017;101:1535-1541.