Observation has long been the gold standard for managing nonproliferative diabetic retinopathy in the absence of diabetic macular edema, even in cases of more severe disease. Treatment with intravitreal anti-vascular endothelial growth factor injections has traditionally been reserved for patients with proliferative DR, but new research from the last decade suggests the ability of prophylactic treatment to regress features of DR in patients without proliferation. The question that remains is whether these clinical benefits outweigh the cost and burden of frequent injections.
To help inform your clinical decision-making when caring for this patient population, in this article, we’ll discuss the significance of the recent trials’ findings, patient selection for early intervention and the potential pros and cons of preventative treatment for severe NPDR. Plus, several retina specialists share their current protocols for managing these patients and whether their approaches have changed in light of new evidence.
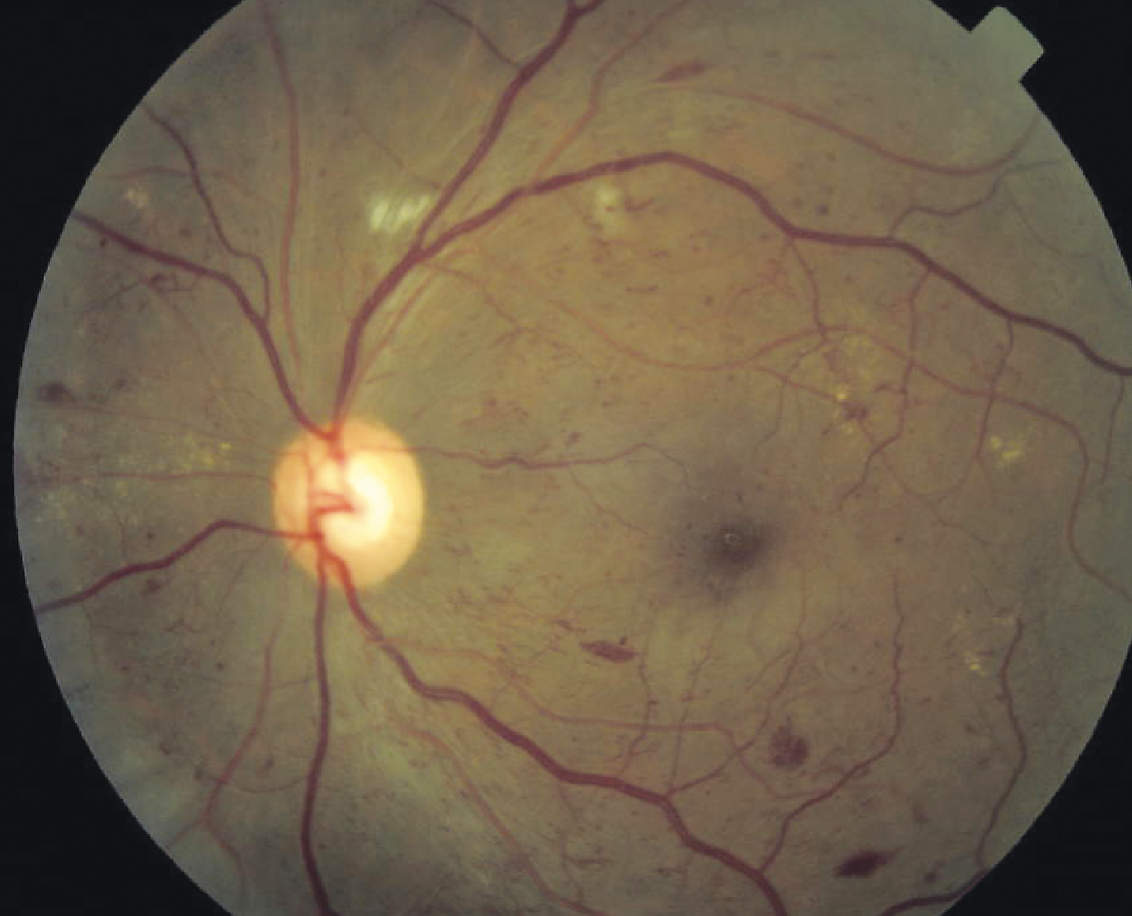 |
| A patient with severe NPDR. (Courtesy Carl Regillo, MD) |
What Current Research Shows
Two trials conducted recently—PANORAMA and Protocol W—investigated the clinical and visual outcomes of administering anti-VEGF to patients with severe NPDR without diabetic macular edema. While these studies are ongoing, so far, the two-year outcomes reported in PANORAMA and four-year outcomes reported in Protocol W suggest that preventative injections may decrease some of the anatomic effects and vision-threatening complications of NPDR; however, neither trial observed that initiating anti-VEGF during this disease stage had a significant effect on visual acuity compared with sham injections.
Let’s delve further into what these two clinical trials observed about the effects of prophylactic anti-VEGF on late-stage NPDR in the absence of DME.
PANORAMA
The Study of the Efficacy and Safety of Intravitreal Aflibercept for the Improvement of Moderately Severe to Severe NPDR, otherwise known as PANORAMA, was a 100-week, double-masked, randomized clinical trial sponsored by Regeneron which aimed to determine whether treating moderately severe to severe NPDR would have a significant effect on disease severity and incidence of vision-threatening complications and center-involved DME. Its results were published in 2021.1
A total of 402 participants (one eye per participant) were recruited from 87 clinics across the United States, Japan and Europe. The cohort was then divided into three groups:
- aflibercept 2q16 group, receiving intravitreal injections of aflibercept, 2 mg, every 16 weeks after three initial monthly doses and one eight-week interval;
- aflibercept 2q8/PRN group, receiving intravitreal injections of aflibercept, 2 mg, every eight weeks after five initial monthly doses, with pro re nata dosing beginning at week 56; and
- the control group, which received sham injections.
At baseline, all participants had a DR Severity Scale level between 47 and 53, no DME and best-corrected visual acuity of 20/40 or better. The main outcomes of the study included the proportion of eyes with at least a two-step improvement in DRSS level and the incidence of vision-threatening complications and center-involved DME from baseline to weeks 24, 52 and 100.
The results showed that at 24 weeks, treatment with aflibercept resulted in a two-step or greater improvement in DRSS level in 58.4 percent of eyes compared with just 6 percent of eyes in the control group. These percentages continued to increase at 52 weeks, with 65.2 percent of eyes in the aflibercept 2q16 group, 79.9 percent of eyes in the aflibercept 2q8/PRN group and 15 percent of eyes in the control group showing at least a two-step improvement in DRSS level. By 100 weeks, these numbers dropped slightly to 62.2 percent of the 2q16 group, 50 percent of the 2q8/PRN group and 12.8 percent of the control group.
The researchers noted in their paper on PANORAMA’s findings that the “outcomes on the DRSS between years one and two emphasize the need for ongoing vascular endothelial growth factor suppression and adherence.”1 Additionally, they reported that, compared with the control group, “the risk of a two-step or greater worsening in DRSS level was significantly reduced by 89 percent at week 52 and 81 percent at week 100 in the aflibercept 2q16 group, and by 100 percent at week 52 and 93 percent at week 100 in the aflibercept 2q8/PRN group.”
Not only did the PANORAMA trial conclude that aflibercept injections may be effective in severe NPDR to help improve disease severity, but the data also revealed it may reduce the risk of vision-threatening complications and/or center-involved DME. At week 100, nearly half of the patients in the control group experienced one or both of these clinical events, whereas closer to one in six patients treated with aflibercept developed vision-threatening complications and/or center-involved DME (16.3 percent of the 2q16 group and 18.7 percent of the 2q8/PRN group).
Of note, the study observed no significant difference in visual acuity in aflibercept-treated patients vs. controls from baseline to two years. A follow-up study analyzing the longer-term results is expected to be published.
PANORAMA’s findings suggest that intravitreal aflibercept injections have a good safety profile in patients with moderately severe to severe NPDR and in some cases may even reduce disease severity and prevent visual complications; however, aside from anatomical outcomes, there are still other factors to consider when deciding whether a patient with severe NPDR will benefit from early intervention, such as cost, treatment burden and effects on quality of life.
.jpg) |
| Right eye of a male in his 40s with persistently poor glycemic control (HbA1c >10%). Fundus photo (top) shows many microaneurysms and dot-blot hemorrhages, with numerous areas of capillary flow loss and intraretinal microvascular abnormalities apparent on swept-source OCT-A (bottom). He progressed to high-risk PDR within two years of follow-up and has since been treated with anti-VEGF and PRP, maintaining 20/20 vision in both eyes after six years of treatment. (Courtesy Ian Han, MD) |
Protocol W
The DRCR.net randomized trial, Protocol W, was conducted with a design similar to that of PANORAMA, but rather than looking at two years of data, it analyzed the four-year outcomes of visual acuity and rates of vision-threatening complications in eyes with moderate to severe NPDR treated with aflibercept vs. sham injection. Its findings were published this past January.2
The clinical trial included 328 total participants (399 eyes) from 64 sites around the United States and Canada with DRSS levels ranging from 43 to 53. Two hundred eyes were randomly assigned to receive 2 mg aflibercept, while 199 eyes received sham injections.
Participants received eight injections over two years, continuing quarterly through four years unless the eye reverted to mild NPDR or better. Aflibercept injections were administered to patients in either group who developed high-risk PDR or center-involved DME with vision loss during the trial. The main study outcomes were the development of PDR or center-involved DME with vision loss (≥10 letters at one visit or ≥5 letters at two consecutive visits) and change in visual acuity (best-corrected ETDRS letter score) from baseline to four years.
The study found the four-year cumulative probability of developing PDR or center-involved DME with vision loss to be 33.9 percent for patients treated with aflibercept and 56.9 percent for those given sham injections. Like PANORAMA, Protocol W also didn’t demonstrate a significant change in visual acuity from baseline to four years (-2.7 letters for aflibercept vs. -2.4 letters for sham injections).
The researchers concluded that, based on Protocol W’s findings, aflibercept may not be warranted as a preventive strategy for patients with NPDR without center-involved DME.
Ian Han, MD, an associate professor in the department of ophthalmology and visual sciences at the University of Iowa Hospital and Clinics, says that the trials’ findings don’t come as a surprise. “The anatomic improvement confirms observations from daily practice as well as prior clinical trials of anti-VEGF therapy for DME (e.g., RISE/RIDE),” he notes. “Because NPDR remains largely defined by fundus features and vascular changes which may not have consequences on visual acuity, it’s not surprising that these trials showed minimal effect on visual acuity despite anti-VEGF therapy.”
.jpg) |
| Left eye of a male in his 40s with consistently good glycemic control (HbA1c of 7%). Fundus photo (left) shows microaneurysms and exudates, with several intraretinal microvascular abnormalities adjacent to patchy areas of nonperfusion seen on FA (right). He’s been followed without treatment for 12 years with no progression to proliferation and stable 20/20 vision in both eyes. (Courtesy Ian Han, MD) |
Considerations for Early Treatment
Although present evidence does show that anti-VEGF may reduce vision-threatening complications and regress anatomic features of severe NPDR, it’s important to consider other factors in play such as cost, treatment burden, patient comorbidities, level of diabetes management and social determinants of health when deciding whether early intervention could be an effective strategy for your patient.
“Debate remains regarding the visual impact or long-term benefits of proactive treatment, as the immediate costs (of the medications, treatment visits, travel back and forth to the clinic) can be very burdensome,” notes Dr. Han.
David Boyer, MD, a partner at Retina Vitreous Associates Medical Group and adjunct clinical professor of ophthalmology at the Keck School of Medicine of the University of Southern California, says that one thing he considers when deciding to treat patients with severe NPDR is how well controlled their diabetes is, as well as if any concomitant conditions are at play.
“If the patient has an HbA1c of seven or below, that individual is likely compliant and will show up to appointments so they can be followed,” Dr. Boyer says. “However, if I have a patient with a very elevated HbA1c, that patient is probably not compliant, and their disease will likely continue to progress.”
For patients with severe retinopathy and no DME, Dr. Boyer notes that he will sometimes choose to perform panretinal photocoagulation, citing the following reasoning: “If patients don’t come back for insurance-related or other reasons, or if their diabetes remains out of control, the laser ensures they at least have some degree of treatment on board and, hopefully, won’t go on to develop tractional detachments or loss of light perception.”
Carl Regillo, MD, chief of the retina service at Wills Eye Hospital in Philadelphia, agrees that “compliance is always an issue with these patients. If you’re treating them, and they don’t show up, they lose the benefit. But, if you’re not treating them, you lose the opportunity to potentially detect and manage these problems earlier on.” He adds, “This patient population is also often in the workforce and juggles several other health issues, which makes it even harder for them to keep up with regular appointments, especially the monthly visits that anti-VEGF therapy requires.”
Missing follow-ups is certainly not uncommon among DR patients. A recent study found that three in four patients with DR experience lapses in care, with even higher rates among black and Hispanic patients.3 Dr. Han notes that “patients who have poor overall systemic control and social determinants of health are perhaps the best candidates for proactive treatment of NPDR, in part because of their high risk for eventual progression to PDR as well as lapses of care.”
Jason Hsu, MD, co-director of retina research at Wills Eye Hospital, assistant professor of clinical ophthalmology at Thomas Jefferson University Hospital in Philadelphia, and a managing partner of Mid Atlantic Retina, adds that when deciding to treat NPDR with anti-VEGF, consider the possibility that “some patients will have a false sense of security and perhaps stop returning due to the belief that a few injections have lasting benefits. However, it doesn’t appear to be the case that anti-VEGF therapy has long-lasting benefits once the injections are paused or stopped.”
Dr. Boyer also makes the point that oftentimes, individuals participating in clinical trials—such as PANORAMA and Protocol W—are more cooperative and likely to adhere to a treatment regime than patients in the real world. “Study patients have people calling them to remind them to come back in for treatment, so those patients do quite well,” he explains. “But in real life, there are studies that show what happens when these patients don’t show up, and it’s disastrous, though it’s less of a disaster if you have PRP present.”
Regarding preventative anti-VEGF therapy for NPDR, Dr. Hsu summarizes the seemingly popular opinion of retina specialists today. “The cost to society as a whole has to be considered when thinking about the pros and cons of a preventative therapy,” he says. “In this case, the studies to date haven’t convinced me that the benefits outweigh the risks and costs.”
Managing NPDR Without DME
Retina specialists have varying approaches to managing patients with moderately severe to severe retinopathy when no edema is present. A few doctors elaborate on theirs below.
Dr. Han explains that if a patient has severe NPDR without DME, “I typically observe the NPDR rather than proactively treat with anti-VEGF therapy for the retinopathy alone.” He notes that he will routinely monitor the patient every few months, performing a “careful dilated fundus examination, with fundus photography to assist in documenting the well-established features of DR.”
For assessing these patients in vivo, Dr. Han points out that “OCT is not as helpful for tracking most features of NPDR. Because these were established in the era of fundus photography and fluorescein angiography, most of the defined features of NPDR are better appreciated using clinical examination or FA (e.g., intraretinal hemorrhages, vascular abnormalities such as venous beading/intraretinal microvascular abnormalities).”
In addition to observation, Dr. Han also notes that he makes it a point to “educate the patient on the clinical findings and encourage their continued vigilance for overall glucose control, as well as optimization of systemic risk factors. Seeing diabetic damage in the eye can often motivate a patient to take better care of their overall health, which benefits them in the long run.”
Dr. Regillo also relies on observation as his primary approach to treating NPDR if no DME is detected. “I closely monitor these patients every three or four months so I can detect any vision-threatening complications at their earliest stages when anti-VEGF will be most effective. Then, I treat as needed,” he notes.
Dr. Hsu, another proponent of observation in NPDR without DME, notes that in addition to more frequently monitoring patients with poor glucose control, “patients with diabetes who are pregnant may also exhibit more rapid progression of retinopathy and need to be monitored very closely.”
There are certain circumstances when Dr. Han says he’ll consider prophylactically treating these patients with PRP. “If a patient has severe or very severe NPDR with poor glycemic control, as well as numerous barriers to access or social determinants of health that may limit their ability to reliably follow up, proactive treatment with PRP may be considered, as recommended many years ago in ETDRS,” he says.
Although Dr. Hsu doesn’t currently use PRP to treat NPDR, he notes that “early treatment of severe NPDR and early PDR (without high-risk characteristics) with PRP may not be unreasonable based on the ETDRS. PRP has been shown to have long-lasting benefits in prevention of severe vision loss. While it may have negative impacts on peripheral vision and night vision, it is rare for patients to become symptomatic unless the PRP is very dense and posteriorly placed.”
Dr. Boyer also considers PRP in some cases of severe NPDR without edema if patients have poor diabetes management and show signs of retinopathy progression on FA.
“I’ll laser the areas adjacent to the nonperfusion and some of the nonperfused areas,” he says. “But, it also depends on what the patient has on the widefield FA to determine whether they even require any treatment. They may have hemorrhages in all four quadrants, or they might have some venous beading, but if I don’t see a great deal of nonperfusion and capillary dropout, I may observe that patient and not treat them at all.”
Dr. Boyer notes that while he personally can’t justify administering anti-VEGF to a patient with severe NPDR without DME at this time, he may consider intervening sooner when longer-acting treatments requiring fewer office visits become available.
Managing NPDR with DME
When it comes to NPDR in the presence of DME, Dr. Boyer says this is when he will initiate treatment with anti-VEGF. For Dr. Regillo, the decision to treat depends on the degree and location of the macular edema.
“I’ll wait until a certain level of center-involved DME starts to affect the vision before I trigger treatment, so if they start to develop DME, I’ll begin following them a little closer,” he says. He adds that he also factors in the patient’s overall health and metabolic control when deciding how often to monitor them. “I’ll follow up more frequently with patients who have poor diabetes management and a higher HbA1c level since they’re more likely to progress.”
Dr. Regillo notes that his approach to treatment in patients with severe NPDR and center-involved DME has shifted since more research has emerged in support of preventative anti-VEGF.
“I usually treat DME till the macula is dry, then I stop, watch and wait, and then treat for recurrences,” he explains. “However, if a patient has more severe NPDR, I’m now more inclined to keep the treatment going and do more of a treat-and-extend approach. That way, the patient is receiving more continuous anti-VEGF therapy, helping to further improve their level of retinopathy and also decrease their risk of recurrences.”
Takeaways
Until longer-term research is published, the choice to proactively treat severe NPDR patients with anti-VEGF, PRP or a combination is for each physician to make on a case-by-case basis.
“The risk of anti-VEGF injections is relatively low, and they’re well tolerated, but the cost-effectiveness and treatment burden of the preventative approach is what’s in question,” summarizes Dr. Regillo.
The ideal management approach should be that which offers your patient the best chance of avoiding vision-threatening complications while also having minimal impact on their quality of life, physicians say. As new therapeutic modalities for DR emerge from the pipeline—such as gene therapy, the updated port delivery system and suprachoroidal injection—the balance of these two treatment goals may become easier to achieve.
Dr. Boyer is a consultant for Genentech, Roche, Regeneron, Bayer, Novartis, Adverum, RegenxBio and Eyepoint. Dr. Regillo consults for and has received research grant support from Genentech, Novartis, Regeneron and Ocuterra. Dr. Hsu is a consultant for IvericBio, Gyroscope Therapeutics and Bausch + Lomb, and receives grant support from Genentech/Roche, IvericBio and Aldeyra Therapeutics. Dr. Han reports no disclosures.
1. Brown DM, Wykoff CC, Boyer D, et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol 2021;139:9:946-955.
2. Maturi RK, Glassman AR, Josic K, et al. Four-year visual outcomes in the Protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy [published correction appears in JAMA. 2023 Mar 28;329:12:1034]. JAMA. 2023;329:5:376-385.
3. Cai CX, Tran D, Tang T, et al. Health disparities in lapses in diabetic retinopathy care. Ophthalmol Sci. March 3, 2023. [Epub ahead of print].



