 |
Real-world scenarios are fraught with the obstacles of daily living and rarely align with carefully monitored clinical trial settings, so the true impact of many treatments often varies. Fortunately, large databases such as the IRIS Registry enable powerful real-world analyses of ocular outcomes. “The IRIS Registry represents about 70 percent of all eye care visits in the United States, so it’s quite representative of what’s actually happening in the real world, post approval,” says study co-author Theodore Leng, MD, FACS, of the Byers Eye Institute. “Additionally, real-world patients are often very different from trial patients who are carefully selected based on the trial’s inclusion/exclusion criteria. Patients in the real world might not qualify for a trial because of their visual acuity or lesion size or comorbid medical conditions. It’s vitally important to use these real-world studies to see what’s actually happening with these medications once they’re out in the wild, so to speak.”
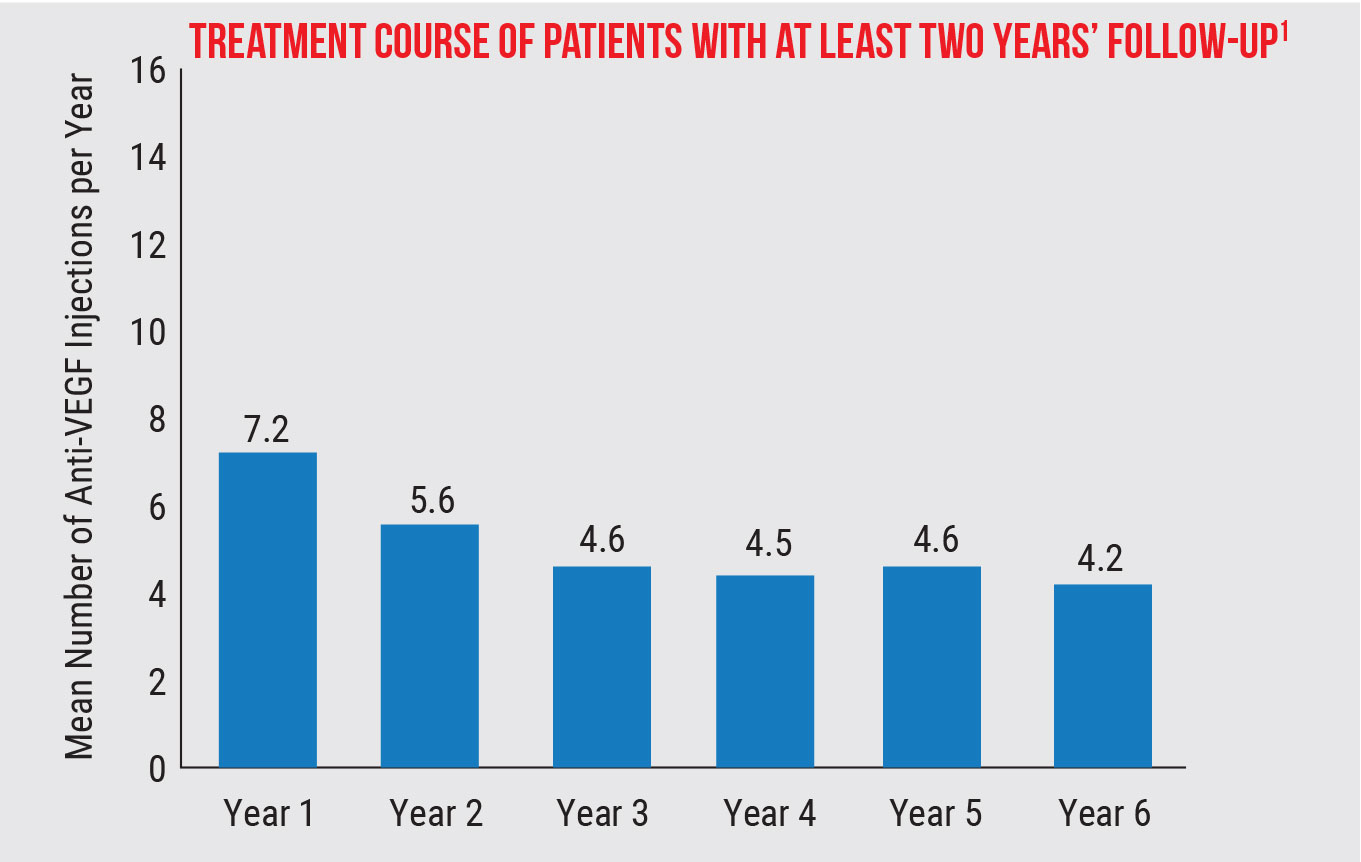
Using the IRIS Registry, researchers analyzed anti-VEGF treatment patterns over a six-year period (the longest to date) and the influence of patient demographics on neovascular age-related macular degeneration outcomes. Their findings showed that in the real world, these patients are undertreated and losing vision in the long term.
The retrospective, noninterventional study included a large cohort of 226,767 patients (254,655 eyes; 160,423 with visual acuity data) with a first anti-VEGF injection and at least two years of follow-up.1 The researchers found that patients experienced a mean visual acuity increase of three ETDRS letters at year one, but this was followed by annual decreases, leading to a net loss from baseline of 4.6 letters after six years. They also reported that patients with longer follow-up had better baseline and follow-up visual acuity.
In the long run, patients received fewer injections. The mean number of injections was 7.2 in year one and 5.6 in year two. This leveled off in years three to six, from 4.2 to 4.6 injections, or about one injection every three months.
A total of 38.8 percent of eyes discontinued treatment and 32.3 percent switched treatment. Adjusted data showed that each additional injection led to a 0.68-letter improvement from baseline to year one, leading the researchers to conclude that multiple injections in a year could be clinically meaningful.
The following risk factors were associated with increased risk of vision loss at year one: older age; male sex; Medicaid insurance; and not being treated by a retina specialist. During follow up, 58.5 percent of patients lost 10 or more letters of vision at least once, and 14.5 percent had sustained poor vision after a median of 3.4 years.
Overall, the study findings indicate that poor adherence to anti-VEGF injections is likely widespread and contributing to vision loss. The researchers pointed out in their paper that new therapies that reduce treatment burden (e.g., via different mechanisms of action, routes of administration or increased durability) may improve visual outcomes.
Dr. Leng adds that in addition to new treatments, clinicians can take some steps to prepare patients for long-term success. “When we start treating patients with nAMD, it’s a good idea to set expectations not only upfront, covering the next few months, but also long-term. Be sure to tell patients that it’s important to keep coming back because there’s a very good chance that they won’t lose any more vision and that their vision may actually improve. The emphasis here is on the long term. I tell patients that this is a treatment for a chronic, lifelong condition, kind of like high blood pressure or diabetes. This isn’t like fixing a broken bone. It’s a long-term journey together. Setting up that expectation early is key.”
Dr. Leng also says that having systems in place in your clinic to reduce the number of patients lost to follow-up can also boost adherence. “Things may be going swimmingly for six months, a year or two years, but every now and then something might happen,” he explains. “A doctor might be out for an emergency, or the patient might be hospitalized or have something else going on their life that forces them to cancel or no-show for their appointment. Sometimes those appointments never get rescheduled, and that’s when patients lose vision. Building some systems into our practice workflows to help prevent that from happening could help.”
1. Wykoff CC, Garmo V, Tabano D, et al. Impact of anti-VEGF treatment and patient characteristics on vision outcomes in nAMD: Up to 6-year analysis of the AAO IRIS Registry. Ophthalmology Science 2023. [Epub ahead of print].
Alternative Postop Regimen Similar to Traditional One
 |
After cataract surgery, the typical regimen of topical antibiotics, corticosteroids and a nonsteroidal anti-inflammatory, sometimes called “triple-drop therapy,” comes with its own set of limitations, including cost, patient adherence issues and the potential for elderly patients to experience physical difficulty administering drops. Due to these factors, alternatives have emerged. While perioperative intracameral injection is quite popular with cataract surgeons, a group of researchers decided to investigate outcomes with intravitreal administration of an antibiotic and a steroid (abbreviated IVAS) coupled with post-op topical NSAID, a regimen known as IVAS-NSAID for short. The antibiotic agent was moxifloxacin and the steroid was triamcinolone.
The retrospective study compared non-infectious outcomes of this method against topical therapy usage.1 In total, 2,143 eyes of 2,143 patients—1,079 receiving IVAS-NSAID and 1,064 receiving TDT—who underwent cataract surgery were included from three diverse study centers in the United States. Best-corrected visual acuity and intraocular pressures were similar between the groups at all time points of one week, one month and six months post-surgery.
A total of 11.6 percent of TDT eyes developed post-op complications, but the rate was even lower in the IVAS-NSAID group—just 6.5 percent. Interestingly, both patients who underwent femtosecond laser–assisted cataract surgery (FLACS) and those with dark irides experienced a higher incidence of persistent corneal edema after IVAS-NSAID. As well, those with dark irides also saw higher incidence of cystoid macular edema and rebound inflammation.
Expanding on these findings, the study authors explained, in a paper for American Journal of Ophthalmology, that “IVAS-NSAID may be considered a safe alternative to topical regimens in non-FLACS and light irides patient populations” while reducing the eye drop burden for most patients.
Similar rates of IOP elevation were seen between both groups, which could be explained for a few reasons. One is that surgeons used a technique of manual wound burping after IVAS injection to confirm acceptable IOP, which was not routinely done in the TDT group. Another likelihood is due to the researchers of this study using lower concentration of triamcinolone (2.25 mg) in the IVAS injection than what is injected for posterior segment inflammation (4 mg), which has been the focus of previous studies that reported higher IOP rates with injection. As well, injection closer to the limbus carries greater propensity for IOP elevation.
Postop inflammatory events were similar, with risk factors of particular concern (prior uveitis, diabetic status) not associated with increased inflammatory risks with IVAS-NSAID; this demonstrates a comparable safety profile to TDT without posing additional risks, the researchers said.
Patients who underwent FLACS and received IVAS-NSAID had higher rates of persistent corneal edema, reflecting previous reports citing increased postop inflammation following FLACS. Similarly, those with dark irides have previously been found to have higher rates and degrees of postoperative inflammation, reflecting the greater rates seen in this study of rebound inflammation, persistent corneal edema and cystoid macular edema.
Due to this association, the researchers suggested that “surgeons may consider avoiding IVAS in patients with dark irides or using additional anti-inflammatory medications (e.g., additional injectable, implantable or topical steroids) to supplement IVAS-NSAID.”
Other than these contraindications, the IVAS-NSAID technique may improve patient compliance and reduce health-care costs while providing a simplified postop medication course, the researchers concluded, stating their belief that “these findings can aid surgeons in developing personalized postoperative regimens for the optimal use of IVAS-NSAID, based on patients’ comorbidities and demographic characteristics.”
1. Mian OT, Asif H, Sandhu U, et al. Non-infectious outcomes of intravitreal antibiotic-steroid injection and topical NSAID vs. triple drop therapy post cataract surgery. Am J Ophthalmol. November 7, 2023. [Epub ahead of print].
Body Position’s Impact on Intraocular Pressure
Fluctuations in intraocular pressure, particularly over a 24-hour period, are a known contributor to glaucoma progression. Given that research has also shown that IOP readings can be affected by posture, glaucoma patients may potentially be able to reduce IOP fluctuations by adjusting their posture throughout the day, as demonstrated by a recent observational study. It also offers clinicians guidance on improving the reliability of in-office readings by being mindful of patients’ posture.
A total of 74 patients (148 eyes) with open-angle glaucoma participated.1 Researchers obtained introacular pressure measurements from patients while they were in the following positions: supine, left lateral decubitus, right lateral decubitus, head tilted downwards position with immediate head-up (transient head tilted downwards), seated, seated with head tilted downwards, standing and walking. Patients held each position for five minutes before IOP readings were taken.
Overall, IOP seemed to decrease as elevation of body position increased. Compared with the seated position, IOP was significantly higher with the three sleeping positions—supine, left lateral decubitus, right lateral decubitus—as well as with the patient’s head tilted downwards.
The study authors theorized in their paper that “the decrease in the vertical distance between the eyes and the heart in three different sleeping postures and the head tilted downwards position could lead to an increase in IOP.” They further explained, “The reduction in gravitational potential energy associated with the drainage of aqueous humor into the heart might contribute to the elevation of intraocular pressure.”
The researchers also observed that the lateral sleeping position showed a higher intraocular pressure than the supine position, which they suggest could be attributed to the fact that “the lateral position requires more gravity to overcome when aqueous humor flows out.”
On the other hand, IOP decreased significantly when standing or walking compared with the seated position (with eyes at primary gaze) or any of the sleeping positions. Additionally, walking decreased intraoacular pressure levels significantly more than standing.
“The act of standing upright or walking is associated with a temporary reduction in central blood volume, leading to a decrease in cardiac output and mean arterial pressure,” the study authors elaborated in their paper. Further supporting the observed pressure decrease during walking, prior research has also shown that aerobic exercise can reduce IOP.
Based on this data, clinicians may consider recommending their open-angle glaucoma patients to sleep in a lateral position and spend more time standing and walking throughout the day to minimize intraocular pressure fluctuations. Additionally, the researchers suggest that during 24-hour IOP monitoring, accurate nighttime measurements should be obtained while patients are in their sleeping postures rather than seated. Lastly, to improve the reliability of in-office intraocular pressure readings, the researchers advise clinicians to perform the measurement after a patient has maintained a seated-level gaze position for at least five minutes.
1. Sang Q, Xin C, Yang D, Mu D, Wang N. Effect of different postures on intraocular pressure in open-angle glaucoma. Ophthalmol Ther. November 4, 2023. [Epub ahead of print].
CORRECTIONS In the November article, “How to Select the Right MIGS for the Job,” the procedure being studied by ViaLase was incorrectly described as femtosecond laser trabeculostomy. The actual name of the procedure is femtosecond laser image-guided high-precision trabeculotomy. Review regrets the error. |




