New research found that more than 70 percent of patients receiving an initial glaucoma evaluation in the United States do not have record of gonioscopy.1
In this retrospective, case-control study, researchers assessed patterns in gonioscopy during initial glaucoma evaluations in the United States. Subjects with a diagnosis of glaucoma suspect, anatomical narrow angle (ANA) or primary/secondary glaucoma were included. Among the 198,995 patients (56 percent female, 44 percent male) in this analysis, 20.4 percent had a recorded gonioscopy on the day of diagnosis and 29.5 percent within six months. There was no gender difference noted in gonioscopy rates.
 |
|
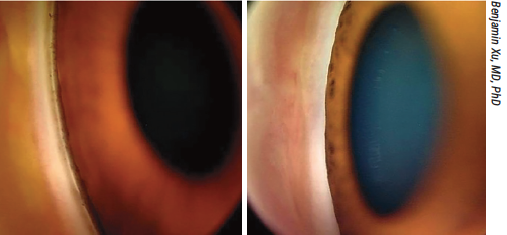
Though gonioscopy is recommended for all patients undergoing a glaucoma evaluation, one study found only around 30 percent of these patients are receiving the exam. Shown here, an open (left) and a closed (right) angle. |
“One thing that struck us as surprising was how low the rate of gonioscopy actually was,” says one of the study’s authors, Benjamin Xu, MD, PhD, of USC’s Keck School of Medicine. “Previous studies had found it was probably closer to 50 percent, but those were in more academic settings. Another study looked at gonioscopy prior to doing glaucoma surgery, so it wasn’t exactly at time of glaucoma evaluation. Those studies had found it was closer to 50 percent of those who should have received gonioscopy had a record of it. The fact that ours was actually lower was a bit surprising and concerning, as well. As the article mentions, the AAO really does recommend that all patients undergoing gluacoma evaluation receive gonioscopy.
“What was also surprising was that some of these patients who were being diagnosed with angle closure or anatomically narrow angles didn’t have a record of gonioscopy,” Dr. Xu continues. “So whether these providers are just assuming based on other exam techniques like van Herick, it’s surprising and leads to the question: Are people just not billing for it? It doesn’t pay a lot, about $20, but are people not aware to bill for it? I will say that most providers are pretty good about being aware of the in-office exam components that reimburse. So I’d find that to be surprising.”
The researchers found several racial distinctions. Multivariate analysis revealed that the odds of recorded gonioscopy within six months of initial evaluation was lower among non-Hispanic white patients; however, it was similar for black and Hispanic patients when compared to their Asian counterparts. Among patients with primary angle closure glaucoma, only 56.6 percent of Asian subjects had a record of gonioscopy, whereas the other racial cohorts all had rates of at least 70 percent or more.
“Primary factors that influence a provider’s decision to perform gonioscopy during glaucoma evaluation include perceived risk of angle closure based on patient demographics (e.g., Asian race or older age) or clinical findings associated with angle closure (e.g., shallow anterior chamber depth or hyperopic refractive error),” the researchers wrote in their recent American Journal of Ophthalmology paper on the work. “If the former was predominant, we should have observed significantly higher rates of recorded gonioscopy among Asians, which we did not.”
“We were happy to see that black and Hispanic patients were as likely to receive gonioscopy as Asians,” says Dr. Xu. “It’s widely known that Asians are at higher risk for angle closure and angle closure glaucoma. In a previous study we conducted, we found that black patients were unfortunately not receiving gonioscopy prior to developing glaucoma. But what this study shows, at least when they’re evaluated for glaucoma, is that they appear as likely to receive gonioscopy.
“It’s also not surprising that non-Hispanic whites are less likely to receive gonioscopy,” Dr. Xu continues, “because they tend to have deeper anterior chambers, so they appear to be less likely to need gonioscopy. However, the recommendation for the first-time glaucoma evaluation is for everyone to receive gonioscopy. So this finding probably indicates that providers are doing gonioscopy selectively.”
One of the reasons for the low rate of gonioscopy is potential anchoring bias, which involves hewing to the first piece of information you’re given on a particular topic. “This has to do with what we’re taught as trainees,” says Dr. Xu. “As ophthalmology residents, the Basic and Clinical Science texts emphasize the importance of Asian race as a risk factor for narrow angles and narrow angle glaucoma. So, if you’re taught that this disease is more prevalent in a subpopulation, you’re more inclined to look for it in that subpopulation. However, by doing this, you’re overlooking the fact that there are many people in other subpopulations that do have the disease. So, that’s one of the challenges in teaching residents or trainees about the risk factors for disease because it creates these anchoring biases.”
Dr. Xu was asked about the possibility that gonioscopy is skipped in some patients due to a combination of other exam factors, such as racial predisposition, in addition to a need to keep a clinic’s patient flow moving.
“Gonioscopy takes time, takes expertise,” Dr. Xu says. “And it can be hard to find time to do it in a busy clinical workflow. That’s why our lab is developing new methods using technology like OCT to try to facilitate or simplify the clinical workflow. Another issue with gonioscopy is it typically has to be performed prior to dilation, so then you have to see them twice. So, it’s true that gonioscopy isn’t convenient for a streamlined clinical workflow, but it’s a very important part of glaucoma evaluation.”
Additionally, the study authors reported that the odds of recorded gonioscopy was also lower among patients over the age of 60, as well as those who lived outside of the Northeast region. “While it remains unclear whether this difference is related to practice or billing patterns, our results are consistent with prior studies that reported insured patients in the Northeast region are more likely to be detected with ANA prior to developing PACG.”
Dr. Xu says they’ve seen this phenomenon before, and there are several possible explanations for it. “We see that diseases are detected more often and outcomes are better in the Northeast region,” he says. “We think this might be due to the density of providers there: You’re more likely to have access to a provider. There’s also a high density of academic centers, so presumably doctors at those centers may be more up-to-date in terms of practice patterns and adhere to the standard of care. They are teaching residents as well, so that may have something to do with it too. There may also be exposure to a more diverse cohort of patients. There may be more specialists in the Northeast, as well.”
When compared to patients with anatomical narrow angle glaucoma, data showed that angle closure glaucoma, secondary glaucoma or open angle glaucoma/suspect patients were less likely to have recorded gonioscopy.
Dr. Xu hopes that maybe the study will help get the word out about the importance of gonioscopy.
“The reason we wrote the article was to remind people that gonioscopy is a crucial element of the glaucoma evaluation,” he says. “Not doing it can lead to misdiagnosis and mistreatment.” He adds, however, that there seems to be some pushback on the importance of gonioscopy, even from trained glaucoma specialists. “One of the reviewers’ comments—this is a top scientist—really shows the depth of this belief that perhaps gonioscopy is optional: ‘What is the evidence of the need for gonioscopy when evaluating a glaucoma patient? Where preferred practice patterns may expect the collection of gonisocopy data, the underlying evidence regarding the need for such data for management of the patient is weak at best. Therefore, It’s unclear to me why clinicians should collect this data in routine glaucoma patients.’
“This was a bit surprising,” comments Dr. Xu, because clearly he’s aware of the preferred practice patterns. Maybe we need more compelling evidence of why not doing gonioscopy could be problematic. But intuitively, the first fork in the glaucoma decision tree when making the diagnosis is: Is it open angle or angle closure? This is because the two are treated differently. And, you have to perform gonioscopy to make that determination. So, there’s a very simple answer to this reviewer’s objections: Gonioscopy is a fundamental aspect of managing the glaucoma. But, here’s a very experienced clinician asking why we need to do it. Perhaps we need to make this point more clearly when either writing preferred practice patterns or teaching trainees. It’s important to emphasize that the clinical management of glaucoma depends on the underlying mechanism and gonioscopy helps us understand that.
“Ultimately, some patients with narrow angles do develop narrow angle glaucoma, which is a higly blinding disease,” Dr. Xu adds. “And, here in the U.S., even though we spend a lot of money on eye care, one out of eight patients is blind in at least one eye from this disease at first diagnosis. So we need to do better and in order to do so, we have to be better about adhering to these clinical guidelines.”
1. Hui LJ, Kristy Y, Khristin I, et al. Patterns and disparities in recorded gonioscopy during initial glaucoma evaluations in the United States. American Journal of Ophthalmology. February 26, 2024 [Epub ahead of print].
RA Leads to Greater Cataract, Glaucoma Risk
Though seemingly unrelated, numerous studies have pointed to potential causal associations that exist between cataract, glaucoma and rheumatoid arthritis. However, it remains unclear whether RA is indeed a directly influencing underlying condition that raises risks for cataract or glaucoma. In a new genetic analysis, study researchers investigated the relationship of these conditions in European and East Asian populations.1
Genome-wide association study (GWAS) summary statistics were collected for cataract from 372,386 individuals and glaucoma from 377,277 individuals in the European population. RA summary data in this population was derived from a meta-analysis of 97,173 samples GWAS. The East Asian study population comprised 212,453 individuals for cataract and glaucoma and 22,515 individuals for RA.
Between eight and 56 single-nucleotide polymorphisms suited for investigation, depending on the condition. After analysis, the study researchers revealed that RA had an increased risk of cataract and glaucoma in the European population. RA only showed a positive association with cataract in the East Asian population. The authors “believe that oxidative stress and local inflammation are responsible for these causal associations,” and they expand upon this statement in their discussion. It should be noted that reverse MR analyses suggested that cataract and glaucoma had no causal effect on RA.
Characterized by inflammatory changes in the synovial membrane of joints and erosive arthritis, RA has more recently gained increasing attention due to oxidative stress, which is thought to be a key player in development of the condition. Both the mitochondria and blood of RA patients have exhibited elevated levels of reactive oxygen species, which is a prominent biomarker of oxidative stress. Subsequently, these species can cause damage to articular cartilage either directly or indirectly, leading to proteoglycan degradation and inhibition of their synthesis. Pathogenesis of cataract is also related to oxidative stress, with an imbalance in the lens’ redox state driven by this stress and contributing to development. As well, oxidative stress accelerates lens epithelial cell loss, also a critical factor in cataract development.
Shifting to other aspects of the disease, RA also involves local inflammation as a central element in its development. Inflammatory factors and chemokines of tumor necrosis factor, interleukins and matrix metalloproteinase are all upregulated in synovial macrophages and dendritic cell subsets in RA patients. With this upregulation, the inflammatory mediators lead to cartilage degradation, bone erosion and accelerated RA development. Inflammation also plays a critical role in glaucoma pathogenesis. The same inflammatory factors of tumor necrosis factor, two different interleukins and matrix metalloproteinase can all promote retinal ganglion cell death—a hallmark of glaucoma development.
The authors are hopeful that their results may “offer guidance in the early prevention of cataract and glaucoma in RA patients and provide some evidence for the RA-induced inflammation on ophthalmic diseases.”
1. Teng M, Wang J, Su X, et al. Causal associations between rheumatoid arthritis, cataract and glaucoma in European and East Asian populations: A bidirectional two-sample mendelian randomization study. PLoS ONE. 2024;19:3:e0299192.
Latest Victory for Ozempic?
Recent studies have demonstrated that a medication commonly prescribed for type 2 diabetes and obesity, glucagon-like peptide-1 receptor agonists (GLP-1RA), plays a role in facilitating retinal neuroprotection, which, in turn, may prevent glaucoma development and progression.
To further explore this hypothesis, researchers in Denmark performed a nationwide, nested case-control study comparing the risk of glaucoma development in individuals with type 2 diabetes being treated with GLP-1RA—a second-line antihyperglycemic medication—vs. those receiving alternative treatments.
Of 264,708 individuals in the Danish database, the researchers identified 1,737 incident glaucoma cases that were matched to 8,685 controls without glaucoma, all of whom were above 21 years old, had no history of glaucoma and were treated with metformin and a second-line antihyperglycemic drug formulation (a GLP-1RA).
Analysis of the data revealed that compared to individuals in the control group, who received treatments other than GLP-1RA, those treated with GLP-1RAs had a lower risk of incident glaucoma (hazard ratio: 0.81). This risk was reduced even further in cases of prolonged treatment extending beyond three years (HR: 0.71), though GLP-1RA treatment for zero to one years (HR: 0.89) and one to three years (HR: 0.85) weren’t significant.
In their paper for Ophthalmology, the study authors explained that their work accomplished two things. “First,” they wrote, “the use of GLP-1RA was associated with a 19-percent decrease in risk of glaucoma. Second, increased exposure to GLP-1RA, especially over extended durations, accentuated this protective association with a duration-response pattern. Notably, with a significant 29-percent risk reduction when looking at three or more years exposure to GLP-1RA.” They added, “Our sensitivity analysis supported the finding of risk reduction when looking at users of GLP-1RA.”
These findings support the possibility of GLP-1RA being an adjunctive therapy to IOP-reducing eye drops in glaucoma management, the authors argue. They advised in their paper, “The lower risk of developing glaucoma among individuals with type 2 diabetes on GLP-1RA warrants further investigation to establish if there is an effect beyond improved glycemic control.”
1. Niazi S, Gnesin F, Thein A-S, et al. Association between glucagon-like peptide-1 receptor agonists and the risk of glaucoma in individuals with type 2 diabetes. Ophthalmology. March 13, 2024. [Epub ahead of print].
ChatGPT Tries Its Hand at Evaluating Images
Researchers recently explored the use of the AI program ChatGPT to assess ophthalmic photos.
The investigators used a publicly available dataset of clinical photos from ophthalmic cases from OCTCases—a medical education platform based from the Department of Ophthalmology and Vision Sciences at University of Toronto—along with clinical multimodal imaging and multiple-choice questions. Of the 137 cases, 136 had multiple-choice questions.
Included in the analysis alongside the 136 cases were 429 total multiple-choice questions and 448 images. The questions were answered at an accuracy of 70 percent overall (n=299). Performance of the chatbot was best on retina questions (77 percent correct) and worst on neuro-ophthalmology questions (58 percent correct). Intermediate performance was seen in categories of ocular oncology (72 percent correct), pediatric ophthalmology (68 percent correct), uveitis (67 percent correct) and glaucoma (61 percent correct). Additionally, ChatGPT was significantly better at answering questions that were non-image based (82 percent) vs. image-based (65 percent).
Expanding on this last point, the researchers, writing in JAMA Ophthalmology,1 wanted to test this prowess in the ophthalmic field. To do this, they relay that the image-processing capabilities are, right now, less robust in niche subspecialties. Despite this shortcoming, the results here do support the potential of ChatGPT to relatively interpret findings from many ophthalmic imaging modalities, researchers say.
Similarly to the subpar analysis of pediatric ophthalmology images, ChatGPT performed worst in the subspecialty of neuro-ophthalmology. The authors explain this may be due to imaging modalities generally used for neuro-ophth vs. the retina, which was the best-performed category. As the retinal category largely consisted of macular OCT and fundus images, “it is plausible that the current release of the chatbot may be better equipped in interpreting more widely used ophthalmic imaging modalities” compared with neuro-ophthalmology’s higher proportion of RNFL and GCC OCT images, they wrote.
This may be the first study using ChatGPT to interpret ophthalmic images, but the chatbot has already been used in the ophthalmic field for other purposes. In a previous study, pitting it against 125 text-based multiple-choice questions used by trainees to prepare for ophthalmology board certification, the previous version of ChatGPT answered 46 percent of these questions correctly. Two months later, this measure rose to 84-percent accuracy. Reflective of this improvement, the authors posit: “Given that this is a novel addition to the chatbot’s platform, we anticipate its performance on image-based questions may increase considerably with time, as was previously observed in our analyses of text-based questions.”
While the performance of ChatGPT in this investigation achieved moderate accuracy, the large learning model is still inferior to previously published AI systems designed for screening or diagnosing retinal pathologies from ophthalmic imaging like OCT scans and fundus images. However, incorporating more robust AI algorithms into the chatbot may further improve their multimodal capabilities.
The authors warn that with this great technology becoming increasingly widespread, “it is imperative to stress their appropriate integration within medical contexts.” However, they look to a future where “as the chatbot’s accuracy increases with time, it may develop the potential to inform clinical decision-making in [eye-care settings] via real-time analysis of ophthalmic cases.”
1. Mihalache A, Huang RS, Popovic MM, et al. Accuracy of an artificial intelligence chatbot’s interpretation of clinical ophthalmic images. JAMA Ophthalmol. February 29, 2024. [Epub ahead of print].
CORRECTIONS In the March feature “How to Succeed with the New Triple Procedure,” Dr. Kourtney Houser’s quote on page 40 should have read: “Any hydrophobic acrylic intraocular lens is safe to use, but I avoid hydrophilic acrylic lenses, as these can calcify and opacify with gas injection.” Dr. Sadeer Hannush’s quote on page 43 should have read: “For example, I’ll strip a diameter of 8.5 mm and I will graft a diameter of 7.75 to 8 mm, so I over-strip by 0.5 to 0.75 mm.” Dr. Hannush’s complete title is “Attending surgeon at Wills Eye Hospital and professor of Ophthalmology at Thomas Jefferson University in Philadelphia.” Review regrets the errors. |




