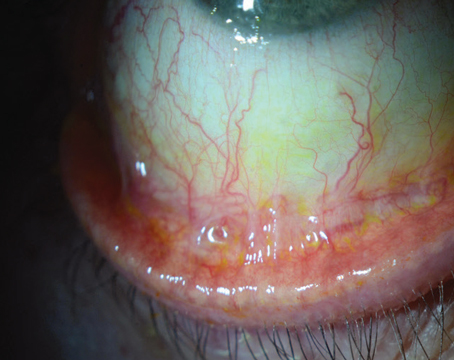
When it’s not noticeable or pronounced, a pterygium may be just a minor annoyance. However, when it compromises vision, or is cosmetically unacceptable, it becomes clinically significant. Here, I’ll review the etiology and diagnosis of pterygia, and outline the best approach to managing them.
Pterygium Backgrounder
Pterygium consists of a triangular, fleshy fold of tissue that extends from the conjunctiva and encroaches onto the cornea. It’s characterized by chronic inflammation, fibrovascular proliferation and tissue invasion. The main risk factors for it are exposure to UV light and advanced age. Pterygia vary in size from small to large, and consist of often aggressive, fibrovascular growths that are more commonly found on the nasal surface of the eye and extend towards the pupil. Vision is often impaired by cosmetic deformity and discomfort and, when vision is affected, surgery is often indicated.
Pterygium Surgery
The key objectives of pterygium surgery are to remove the pterygium completely and achieve rapid post-surgical corneal re-epithelialization with minimal inflammation and scarring. Ultimately, these objectives should reduce the risk of recurrence, achieve the optimal cosmetic outcome and visual acuity, and leave the patient with no discomfort. Although, relatively speaking, the surgical excision of the pterygium is not too difficult, there remains an unpredictable rate of pterygium recurrence, and there’s no single surgical technique that’s universally successful. This is an ever-increasing problem, as today’s patients have higher expectations for optimal vision and cosmetic outcome with no postoperative discomfort.
Corneal surgeons have explored many different surgical techniques in order to improve outcomes and reduce pterygium recurrence. The overall recurrence rate after pterygium surgery has significantly declined from as high as 30 to 82 percent over the past few decades to less than 10 percent now. While the objectives of the surgery remain straightforward, the means of executing such objectives is still under great debate. It’s known that simple excision involving an uncovered bare sclera can yield recurrence rates as high as 88 percent. To reduce the recurrence, tissue grafts are placed in the region of scleral exposure. However, one of the key surgical decisions is whether to use conjunctival autograft or amniotic membrane graft after pterygium excision.
Amniotic Membrane Explained
Amniotic membrane consists of a simple epithelial basement membrane and an avascular stromal layer. The basement membrane of the amniotic membrane has been shown to promote epithelial adhesion, growth and differentiation. The tissue also has anti-inflammatory properties: Amniotic membrane has been shown to promote apoptosis of activated inflammatory cells, promote phagocytosis and increase the expression of anti-inflammatory cytokines such as IL-10. In addition, the tissue has been shown to prevent proliferation and myofibroblast differentiation and aid the eye’s anti-scarring efforts by suppressing TGF-beta signaling. Also, it’s been shown that an inflammatory insult may activate the transformation of pterygial fibroblasts into an invasive phenotype. Therefore, by reducing inflammation and scarring with amniotic membrane, we may decrease the risk of pterygium recurrence. Theoretically, these properties make it an ideal candidate for a tissue graft during pterygium surgery.
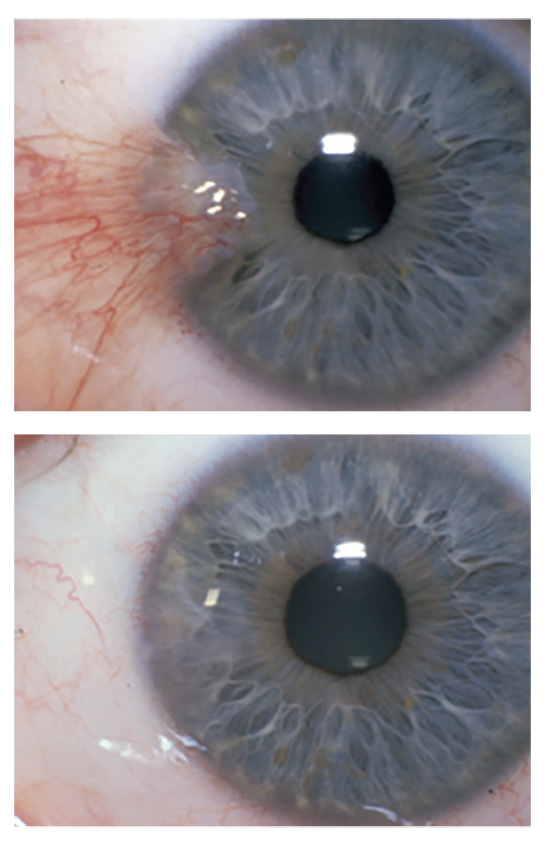 |
| Figure 1. Pterygium excision with amniotic membrane transplantion, before and after surgery. |
Graft Pros and Cons
Amniotic membrane is obtained from healthy, caesarian section, full-term births. It’s then processed/manufactured to decrease the risk of disease transmission and to remove all blood and antigenic components, thereby improving the clinical performance and safety of the allografts. As with any allograft, such as bone or cornea, amniotic membrane can be processed in several different ways. Ideally, the tissue processing method should preserve both the structural integrity of the tissue as well as the biological activity within the matrix. In ophthalmology, the two most common types of amniotic membrane are tissue processed using either dehydration or cryopreservation techniques.
The cryopreservation process involves quickly freezing the amniotic tissue, which has been shown to retain the native architecture of the amniotic membrane matrix and the key biological activity of the tissue. Dehydration, on the other hand, involves high-temperature processing to remove the water content of the tissue; this has been shown to potentially cause protein denaturation, loss of function and possibly irreparable damage to the tissue, with the possible loss of active components and, hence, their associated biological functions.1 Based on these data, cryopreserved amniotic membrane is usually a preferred choice for clinical use in ophthalmology and pterygium surgery.
The advantage of using amniotic membrane rather than conjunctival autograft is that it doesn’t require the use of an additional surgery site where the autograft is harvested which can, in some cases, lead to donor-site morbidity and can complicate future surgeries.2,3 Autografting can also lead to further discomfort and even pain for some patients, whereas amniotic membrane has been shown to have an anti-inflammatory and analgesic component that reduces this effect.
Recently, Ronald Rosen, MD, of Panorama City, California, performed a retrospective study of 556 eyes that underwent pterygium excision with the adjunctive use of cryopreserved amniotic membrane and intraoperative mitomycin-C. His results showed a low recurrence rate of 5.8 percent over a 17-month period.4 Another group of researchers also retrospectively evaluated the use of amniotic membrane, conjunctival autograft or topical 0.02% MMC after pterygium excision. Results showed an insignificantly decreased recurrence rate using amniotic membrane (3.8 percent) or MMC (3.7 percent) versus conjunctival autograft (5.4 percent).5
Surgical Technique
The technique of amniotic membrane transplantation during pterygium surgery involves either peeling the abnormal tissue from the corneal side towards the conjunctiva or from the conjunctival side towards the cornea without damaging the underlying corneal tissue. The pterygium head may be bluntly dissected from the corneal surface by first cutting with scissors at the corneal-limbal junction to separate the head and then by dissecting the remaining head from the corneal surface, using caution to not remove any corneal stromal tissue. The corneal-limbal region is made smooth with a motorized burr, straight crescent blade or a #64 blade. Relaxing incisions are then made radially along both the superior and inferior bulbar quadrants toward the fornix so as to further allow the pterygial head conjunctival tissue to recess to a tension-free position. Adjacent residual pterygial tissue is also excised and the gap is closed. Tenon’s fibrovascular tissue is also excised. Abnormal fibrovascular tissue can be differentiated from healthy Tenon’s by paying attention to the tissue color, texture, stickiness and vascularity. Abnormal fibrovascular tissue is soft, stretchable, red and vascular, while normal Tenon’s appears as dense white fascia.
The cryopreserved amniotic membrane is then cut to fit over the defect and secured using fibrin glue with the “sticky” side down. The sticky side refers to the stromal side of the amniotic membrane; this is known to be stickier than the opposite basement membrane side which, as previously mentioned, promotes epithelial adhesion and rapid healing. Naturally, the stromal side of the graft is adhered to cellulose paper in the product packaging, but you can use a cellulose sponge to determine which side is which.
During graft placement, it’s important to move the tissue toward the conjunctival-Tenon’s gap and place it under the surrounding conjunctival tissue to occupy space and prevent pterygium recurrence. Fibrin glue is then applied and a Weck-Cel sponge is used to squeegee the graft on the sclera and remove any excess glue. Two layers of cryopreserved amniotic membrane can also be used to cover the rectus muscle insertion, as well as the bare sclera. The graft is placed over the bare sclera to cover this region and the muscle sheath, depending on the extent of the surgical dissection, in order to prevent damage and any postop restriction of ocular motility.
Postop, the eye is patched and shielded. Topical prednisolone is prescribed q.i.d. for two months in a tapering dosage, and an antibiotic is used q.i.d. for a week. The patient is usually examined at day one, one week, one month, three months, six months, 12 months and then annually after that.
Overall, this technique allows a more controlled surgical strategy that achieves a predictable outcome with optimal cosmetic results, good patient satisfaction and comfort, and a low recurrence rate. It’s also less tedious and time-consuming than other techniques, because it obviates the need for preparing a conjunctival autograft in these cases. REVIEW
Dr. John is a clinical associate professor at Loyola University at Chicago and is in private practice in Oak Brook, Tinley Park and Oak Lawn, Illinois. He can be reached at 708-429-2223; email: tjcornea@gmail.com.
1. Cooke M, Tan EK, Mandrycky C, et al. Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. J Wound Care 2014;23:10:465-476.
2. Prabhasawat P, Barton K, Burkett G, et al. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 1997;104:974.
3. Solomon A, Pires RTF, Tseng SCG. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 2001;108:449–460.
4. Rosen R. Amniotic Membrane Grafts to Reduce Pterygium Recurrence. Cornea 2018;37:2:189-193.
5. Ma DHK, See LC, Liau SB, et al. Amniotic membrane graft for primary pterygium: Comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol 2000;84:973–978.